Synthesis of Fused-oxazines from Cyclic Ketoximes via α-Nitrosoalkenes
Santosh L. Gaonkar 1,2 and K. M. Lokanatha Rai2
1Department of Chemistry, Manipal Institute of Technology, Manipal University, Manipal-576104, India.
2Department of Studies in Chemistry, University of Mysore, Manasagangotri, Mysore-570 006, India.
Corresponding Author E-mail: gaonkarslg@rediffmail.com
DOI : http://dx.doi.org/10.13005/ojc/330238
Article Received on : March 03, 2017
Article Accepted on : April 04, 2017
Cyclic ketoximes having a α-methylene group on reaction with chloramine-T followed by treatment with triethylamine generate α-nitrosoalkenes via α-chloronitroso intermediates, which are further treated with alkenes to give fused-oxazines.
KEYWORDS:Fused-oxazines; α-nitrosoalkenes; Chloramine-T
Download this article as:| Copy the following to cite this article: Gaonkar S. L, Rai K. M. L.Synthesis of Fused-oxazines from Cyclic Ketoximes via α-Nitrosoalkenes. Orient J Chem 2017;33(2). |
| Copy the following to cite this URL: Gaonkar S. L, Rai K. M. L.Synthesis of Fused-oxazines from Cyclic Ketoximes via α-Nitrosoalkenes. Orient J Chem 2017;33(2). Available from: http://www.orientjchem.org/?p=31580 |
Introduction
1,2-Oxazine and their fused counterparts received interest in these years due to their diverse synthetic and pharmacological importance. 1,2-Oxazine derivatives are useful synthetic building blocks in heterocyclic chemistry. In our preceding report1, we have disclosed the method to obtain α-nitrosoalkenes from ketoximes having α-methylene group and their cycloaddition with olefinic compounds to 1,2-oxazines. α-Nitrosoalkenes behave like dienes (4п-elecron component) in Hetero-Diels-Alder reaction. Further to our research on oxazines and their counterparts, we are reporting the synthesis of α-nitrosoalkenes from cyclic ketoximes bearing a α-methylene group and their cycloaddition with dienophiles to yield fused-oxazines, which are having huge of synthetic applications 2,3 and biological use as therapeutic agents4-7. Generation of α-nitrosoalkenes is quite difficult. Usually they are reactive intermediates and unstable, hence they are only generated in situ2.
In our prior work chloramine-T was used extensively for the synthesis of α-nitrosoolefins1, azoalkenes8-9, nitrile oxides10, nitrile imines11 etc., which undergo cycloaddition with active dienes yield bioactive heterocycles. During the studies, cyclohexanone oxime was reacted with chloramine-T formed a blue colour indicates the formation of chloro-nitroso intermediate, which produces 1-nitroso-cyclohexene on treatment with a base. With this achievement, we are now reporting a new method for the conversion of cyclic ketoximes heaving an active methylene group into α-chloronitroso intermediates, which are appropriate for in situ formation of α-nitrosoalkenes which acts as dienes and undergo [4+2] cycloaddition with dienophiles.
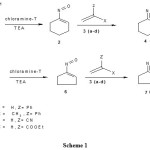 |
Scheme 1 Click here to View scheme |
Results and Discussion
Cyclic ketoximes having a a-hydrogen atom on treatment with chloramine-T followed by treatment with a base are oxidized to a-nitroso alkenes which undergo Hetero-Diels-Alder reaction with olefinic compounds to produce fused-oxazines in good yield. Typically, the reaction was performed by boiling an equimolar mixture of cyclic ketoxime and chloramine-T in ethyl alcohol followed by addition of a base and an alkene. Overall fused-1,2-oxazines are synthesized in good yield as indicated in the scheme 1.
1HNMR, 13CNMR, Mass spectral analyses and elemental analyses established the structure of the fused-oxazines synthesized. The cycloaddition reaction was regioselective. Proton NMR spectrum of fused-oxazine 4 (when X=H) showed dd around d 3.6-4.4 ppm which is due to H3 and triplet in the region d 1.70-1.90 ppm is the signal for H4. Where as in fused-oxazine 4 (when X=CH3), no signal observed for H3 in the above said region, while a new signal was appeared at d 1.69 ppm designates methyl group and H4 observed in the region d 1.70-1.90 ppm as doublet.
13C NMR spectrum of all fused-oxazines gave expected signals for the newly formed ring carbons. For instance, in fused-oxazine 4 (when X=H) the peak due to C3 observed around d 54-67 ppm while 4 (when X=CH3) seen around d 62-69 ppm and a new signal was observed around d 23-26 ppm indicative of the methyl group. The signal in oxazine 4c at d 119 ppm is indicative of the CN group. The stable molecular ion peaks were seen in the Mass spectrum which supports the structure of the newly formed oxazines. The product formation further confirmed by elemental analyses. 1,2-Oxazines and their fused counterparts are potentially very useful heterocycles in synthetic chemistry, and act as useful building blocks for the construction of complex and new heterocyclic compounds.
 |
Scheme 2 Click here to View Scheme |
In summary we have established that fused-oxazines can be produced by the reaction of cyclic ketoximes having a a-methylene group with olefinic compounds in the presence of chloramines-T and a base in good yield.
Experimental
1H NMR and 13C NMR spectra were taken at 400 MHz and 100 MHz respectively on Bruker Avance 400 spectrometer using DMSO-d6 or CDCl3 as solvents and TMS as internal standard. The chemical shifts are stated in d ppm downfield shift from TMS. Mass spectrum was recorded on a Finnigan 4021 mass spectrometer using ionizing energy of 35 ev. Elemental analyses were performed using Vario-EL elemental analyzer. Reactions were checked for the completion by thin layer chromatography (TLC) on precoated silica gel plates using chloroform-ethyl acetate (7:3) as eluent.
Representative process for the preparation of 3-Phenyl-4,4a,5,6,7,8-hexahydro-3H-benzo[c][1,2]oxazine 4a
A mixture of cyclohexanone oxime 1 (1.0 g, 8.85 mmol) and chloramine-T. 3H20 (2.51 g, 8.93 mmol) in ethyl alcohol (6 ml) were boiled for 1 hour. The reaction mass was cooled to rt. and triethylamine (1 ml) was added. The reaction mixture was further stirred for 30 minutes at rt. 3a (0.94 g, 9.03 mmol) dissolved in ethyl alcohol (3 ml) was then added to the above reaction mass and was further boiled 2 hr. After the completion of the reaction, ethyl alcohol was removed under vacuum and the residue left behind was extracted with chloroform (2 X 20 ml). The combined chloroform extract was washed with H20 (15 ml), with dilute NaOH solution (2 ´10 ml) and dried over anhydrous magnesium sulfate. Chloroform was removed under vacuum and the oily mass left behind was purified by column chromatography (CHCl3:EtOAc, 8:2) to give 4a as a pale yellow oily product to yield 1.25 g (66%); 1HNMR (CDCl3, 400 MHz): d 1.24-1.31 (m, 4H, 2 X -CH2), 1.45-1.55 (m, 5H, 2 X -CH2 and -CH), 1.89 (t, 2H, -CH2), 4.61 (dd, 1H, J= 9.7 Hz & 2.5 Hz, -CH), 7.31 (s, 5H, ArH); 13C NMR (CDCl3): d 24.4, 26.8, 29.5, 30.9, 32.6, 35.4, 81.2, 125.1, 128.4, 141.9, 162.5; MS (relative abundance) m/z: 215 (M+,8%), 138, 120, 95 (100%), 77. C, H and N. Calcd. for C14H17NO: C, 78.10; H, 7.96; N, 6.51 %. Found: C, 78.19; H, 8.04; N, 6.43 %.
3-Methyl-3-phenyl-4,4a,5,6,7,8-hexahydro-3H-benzo[c][1,2]oxazine 4b
The product was synthesized from 1 (1.0 g, 8.85 mmol), 3b (1.06 g, 8.98 mmol), chloramine-T.3 H20 (2.51 g, 8.93 mmol) and TEA to give yellow oily product to yield 1.31 g (65%); 1H NMR CDCl3: d 1.26-1.35 (m, 4H, 2XCH2), 1.45-1.55 (m, 5H, 2XCH2 and CH), 1.71 (s, 3H, CH3), 1.93 (d, 2H, CH2), 7.41 (s, 5H, ArH); 13C NMR (CDCl3): d 23.6, 26.1, 29.2, 30.7, 31.5, 32.9, 34.2, 74.6, 125.9, 128.5, 129.0, 139.1, 162.2; MS (relative abundance) m/z: 229 (M+,12%), 152, 134, 95 (100%), 77. C, H and N. Calcd. for C15H19NO: C, 78.56; H, 8.35; N, 6.11 %. Found: C, 78.50; H, 8.41; N, 6.03%.
4,4a,5,6,7,8-Hexahydro-3H-benzo[c][1,2]oxazine-3-carbonitrile 4c
The product was synthesized from 1 (1.0 g, 8.85 mmol), 3c (0.48 g, 9.05 mmol), chloramine-T.3 H20 (2.51 g, 8.93 mmol) and TEA as a yellow oily product to yield 1.03 g (71%); 1H NMR CDCl3: d 1.28-1.34 (m, 4H, 2XCH2), 1.42-1.51 (m, 5H, 2XCH2 and CH), 2.12-2.17 (m, 2H, CH2), 4.69 (dd, 1H, CH); 13C NMR (CDCl3): d 23.2, 26.1, 30.9, 31.3, 34.3, 35.5, 70.3, 118.2, 162.2; MS (relative abundance) m/z: 164 (M+, 13%), 163, 136, 95 (100%), 69. C, H and N. Calcd. for C9H12N2O: C, 65.83; H, 7.37; N, 17.06 %. Found: C, 65.89; H, 7.30; N, 17.11 %.
4,4a,5,6,7,8-Hexahydro-3H-benzo[c][1,2]oxazine-3-carboxylic acid ethyl ester 4d
The product was synthesized from 1 (1.0 g, 8.85 mmol), 3d (0.90 g, 9.0 mmol), chloramine-T.3 H20 (2.51 g, 8.93 mmol) and TEA as a pale yellow oily product to yield 0.48 g (72%); 1H NMR CDCl3: d 1.29 (t, 3H, CH3), 1.35-1.44 (m, 8H, CH2), 1.55-161 (m, 1H, CH), 2.22 (t, 2H, CH2), 4.28 (dd, 1H, CH), 4.69 (q, 2H, CH2); 13C NMR CDCl3: d 12.6, 27.3, 29.4, 30.8, 33.0, 35.3, 61.1, 83.2, 163.2, 174.3; MS (relative abundance) m/z: 211 (M+, 22%), 166 (100%), 138, 95, 71. C, H and N. Calcd. for C11H17NO3: C, 62.54; H, 8.11; N, 6.63 %. Found: C, 62.48; H, 8.19; N, 6.56 %.
3-Phenyl-3,4,4a,5,6,7-hexahydro-cyclopenta[c][1,2]oxazine 7a
The product was synthesized from 5 (1.0 g, 10.10 mmol), 3a (1.07 g, 10.28 mmol), chloramine-T.3 H20 (2.85 g, 10.15 mmol) and TEA to give a pale yellow oily product to yield 0.48 g (64%); 1H NMR CDCl3: d 1.33 (t, 2H, CH2), 1.46-1.55 (m, 5H, 2XCH2 and CH), 2.01 (t, 2H, CH2), 4.42 (dd, 1H, CH), 7.39 (s, 5H, ArH); 13C NMR CDCl3: d 26.2, 27.9, 32.6, 36.5, 41.2, 78.4, 125.8, 128.9, 129.2, 140.1, 161.0; MS (relative abundance) m/z: 201 (M+, 11%), 124, 120, 81 (100%), 77. C, H and N. Calcd. for C13H15NO: C, 77.58; H, 7.51; N, 6.96 %. Found: C 77.48; H, 7.59; N, 6.91%.
3-Methyl-3-phenyl-3,4,4a,5,6,7-hexahydro-cyclopenta[c][1,2]oxazine 7b
The product was synthesized from 5 (1.0 g, 10.1 mmol), 3b (1.20 g, 10.16 mmol), chloramine-T.3 H20 (2.85 g, 10.15 mmol) and TEA to give yellow oily product to yield 0.48 g (67%); 1H NMR CDCl3: d 1.34 (t, 2H, CH2), 1.48-1.55 (m, 4H, 2XCH2), 1.64 (s, 3H, CH3), 1.64-1.68 (m, 1H, CH), 1.98 (d, 2H, CH2), 7.35 (s, 5H, ArH); 13C NMR CDCl3: d 24.2, 26.4, 33.3, 36.3, 38.9, 79.2, 125.3, 128.4, 128.8, 140.7, 162.3; MS (relative abundance) m/z: 215 (M+, 9%), 138, 134, 81 (100%), 77. C, H and N. Calcd. for C14H17NO: C, 78.10; H, 7.96; N, 6.51 %. Found: C, 78.19; H, 7.90; N, 6.58%.
3,4,4a,5,6,7-hexahydro-cyclopenta[c][1,2]oxazine-3-carbonitrile 7c
The product was synthesized from 5 (1.0 g, 10.1 mmol), 3c (0.55 g, 10.37 mmol), chloramine-T.3 H20 (2.85 g, 10.15 mmol) and TEA as pale brown oily product to yield 0.48 g (69%); 1H NMR CDCl3: d 1.39 (t, 3H, CH2), 1.51-1.60 (m, 5H, 2XCH2 and CH), 1.99 (t, 2H, CH2), 4.79 (dd, 1H, CH); 13C NMR CDCl3: d 22.2, 27.8, 32.9, 37.6, 41.1, 71.2, 118.3, 161.5; MS (relative abundance) m/z: 150 (M+, 10%), 149, 122, 81 (100%), 69. C, H and N. Calcd. for C8H10N2O: C, 63.98; H, 6.71; N, 18.65 %. Found: C, 63.90; H, 6.77; N, 18.69 %.
2-Phenyl-3,4,4a,5,6,7-hexahydro-2H-cyclopenta[c]pyridazine-3-carboxylic acid ethyl ester 7d
The product was synthesized from 5 (1.0 g, 10.1 mmol), 3d (1.03 g, 10.30 mmol), chloramine-T.3 H20 (2.85 g, 10.15 mmol) and TEA as a yellow oily mass to yield 0.48 g (68%); 1H NMR CDCl3: d 1.29 (t, 3H, CH3), 1.39-1.52 (m, 6H, 3XCH2), 1.55-1.63 (m, 1H, CH), 1.954-2.01 (m, 2H, CH2), 4.29 (dd, 1H, CH), 4.73 (q, 2H, CH2); 13C NMR CDCl3: d 14.3, 21.3, 26.2, 30.8, 31.2, 33.9, 35.6, 61.2, 84.2, 161.2, 173.6; MS (relative abundance) m/z: 197 (M+, 11%), 197, 152 (100%), 124, 116, 81, 71. C, H and N. Calcd. for C10H15NO3: C, 60.90; H, 7.67; N, 7.10 %. Found: C, 60.99; H, 7.60; N, 7.06 %.
Conclusion
We have established a simple and effective method for the synthesis of fused-oxazines form cyclic ketoximes by Heter-Diels-Alder cycloaddition of in situ generated a-nitroso olefins with various alkenes.
References
- Gaonkar, S. L.; and Rai, K. M. L. J. Heterocyclic Chem. 2005, 42, 877.
- Faragher, R.; Gilchrist, T. L. J. Chem. Soc. Perkin Trans. I , 1979, 249.
- Ito, S.; Saito, I. Tetrahedron Lett. 2005, 46, 5969.
- Polanc, S. J. Heterocyclic Chem. 2005, 42, 401.
- Liyebris, C.; Martinsson, J.; Williams, L. T. M.; Barker, E.; James, E. S.; Duffy; Nygren A.; James, S. Bioorg. Med.Chem. 2002, 10, 3197.
- Bekhit, A. A. Boll Chim Farm. 2001, 140, 243.
- Osborn H. M. I.; Coisson, D. Mini-Rev. Org. Chem. 2004, 1, 41.
- Gaonkar, S. L.; Rai, K. M. L. Tetrahedron Lett. 2005, 46, 5969.
- Gaonkar, S. L.; Rai, K. M. L. J. Heterocyclic Chem. 2015, 52, 1346.
- Gaonkar, S. L.; Rai, K. M. L.; Prabhuswamy, B. Med. Chem. Res. 2007, 15, 407.
- Gaonkar, S. L.; Rai, K. M. L.; Prabhuswamy, Eur. J. Med. Chem. 2006, 41, 841.

This work is licensed under a Creative Commons Attribution 4.0 International License.









