Synthesis and Characterization New Polyurethane Membrane From Hydroxylated Rubber Seed Oil
Marlina1, Saiful1, Rahmi1, Sitti Saleha1 and Salfauqi Nurman2
1Chemistry Department of Mathematics and Sciences Faculty, Syiah Kuala University, Banda Aceh, Indonesia.
2Agriculture Industrial Technology Department of Agriculture Technology Faculty, Serambi MekkahUniversity, Banda Aceh, Indonesia.
Corresponding Author E-mail: marlina_rachman@yahoo.com
DOI : http://dx.doi.org/10.13005/ojc/330122
New polyurethane membrane from hydroxylated rubber seed oil has been preparedin this study. Hydroxylated process of rubber seed oil improved 135.30% of hydroxyl number and decreased 73.91% of iodine number of rubber seed oil. Optimum polyurethane membrane was obtained at the composition of hydroxylated rubber seed oil:hexamethylene-1,6-diisocyanate was 5:7 v/w. The membrane was homogeneous, dry, smooth, slightly stiff and brownish yellow. The flux and rejection factor of the membrane were 0,544L / m2.h.bar and 98.86%, respectively. FTIR spectra indicated the formation of urethane bond (N-H at 3308 cm-1). Thermal analysis showed two stages of decomposition at 223oC and 386oC. The membrane exhibited a strong and elastic properties with a tensile strength of 1.13 kgf/mm2 and elongation 215.36%.
KEYWORDS:Rubber seed oil; Hexamethylene-1,6-diisocyanate; Synthesis; Polyurethane membrane; Characterization
Download this article as:| Copy the following to cite this article: Marlina M, Saiful S, Rahmi R, Saleha S, Nurman S. Synthesis and Characterization New Polyurethane Membrane From Hydroxylated Rubber Seed Oil. Orient J Chem 2017;33(1). |
| Copy the following to cite this URL: Marlina M, Saiful S, Rahmi R, Saleha S, Nurman S. Synthesis and Characterization New Polyurethane Membrane From Hydroxylated Rubber Seed Oil. Orient J Chem 2017;33(1). Available from: http://www.orientjchem.org/?p=27855 |
Introduction
Indonesia is an agricultural country which has a wide range of biological, one of them is rubber plant (Heveabrasiliensis)1. The products of rubber plant are only put emphasis on the processing of latex and trunks, while other products such as its seed has not received more attention. Rubber seeds are the byproducts of rubber plantations which can be utilized for biodiesel2,3. To improve the diversification of plant products, an effort is needed to increase the added value of these plants.
Rubber seed contains 40.76% (w/w) of oil. Rubber seed oil contains 38.59% of oleic acid, 41.06% of linoleic acid, 10.43% of stearic acid and 9.92% of palmitic acid. Rubber seed oil has a hydroxy number 40.33 mgKOH/g and 154.05 gI2/g of iodine number4. Rubber seed oil has a color (lovinbond) 22R; 23,2Y, 0.916 of specific gravity (30 ° C), 43.62 mg KOH/g of acid number, free fatty acids 21.4, 202.91 mg KOH/g saponification number and 136.21 g I2/100 g of iodine numbers5.
Hydroxylation process is the addition of a hydroxy group into a compound by performing addition -OH group to the double bond6. The reaction takes place in two stages, where the first stage is protonated alkene to produce a carbocation. The second stage is nucleophilic addition to the carbocation. Both of reactions take place according to Markovnikov rule. Hydroxylation process in vegetable oils can increase the concentration of hydroxyl6.
In our previous research, polyurethane membranes have been prepared by using vegetable oil. The free fatty acids and oxidized fatty acids of castor oil were used as a source of hydroxyl and 2,4-toluene diisocyanate (TDI) as the source of isocyanate7. polyurethane membrane of rubber seed oil with HMDI have to be synthesized, the resulting membrane has a homogeneous, dry, elastic, brownish yellow, wavy with the flux and rejection factor were 0544 L/m2.h.bar and of 100%, respectively4. Therefore, in this study hydroxylated rubber seed oil and hexamethylene-1,6-diisocyanate (HMDI) were used for polyurethane membrane preparation.
Materials and Methods
Materials
The raw material was rubber seed taken from the plantation residents in the village Gunong Kleng, Meureubo, West Aceh, Aceh Province, Indonesia. Chemicals used were hexamethylene-1,6-diisocyanate (HMDI) purchased from Merck, chloroform, acetic acid, KOH, KI, Na2S2O3, starch indicator, n-hexane, NaCl, H2SO4, and methanol were purchased from Sigma–Aldrich.
Instrumentation
FTIR spectra of rubber seed oil and polyurethane membrane used (Agilent resolution pro cary 630 FTIR spectrometer), membrane morphology viewed using SEM (Tabletop microscope 3000), thermal analysis of polyurethane membrane using TGA and DTA (SDT Q600) and mechanical strength of the membrane was tested with (Control computer series 10-100 KN)
Rubber seed oil extraction process
Rubber seeds were cleaned, peeled, dried at room temperature and crushed into powder. Rubber oil extraction was carried out by n-hexane at 80° C for 4 hours. A rotary evaporator was used to separate the rubber seed oil and n-hexane at 60 ° C for 30 minutes.
Hydroxylation process of rubber seed oil
25 mL of rubber seed oil was added to 2.5 mL of H2SO4 35%, then stirred for 90 minutes, and then chloroform was added to separate the remaining water phase. 5 grams of anhydrous Na2SO4 was added to the organic phase and then filtered. The filtrate obtained was evaporated using a rotary evaporator and the oil obtained was characterized.
Preparation of polyurethane membrane
5 mL of hydroxylated rubber seed oil and 1,6-hexamethylene diisocyanate (with a series of composition) were mixtured by using a magnetic stirrer at a temperature of 90-100°C for 60 min. Then poured in a petri dish, cured an oven at a temperature of 115-120°C for 8 hours. Once the membrane was formed, then the membrane was released in the flowing water with the aid of a spatula.
Result and Discussiong
Characterization of hydroxylated rubber seed oil
Hydroxylation process of rubber seed oil was conducted by using water in acidic solutions. H+ as a catalyst break the double bond of rubber seed oil resulting in the addition reaction with -OH groups from water. The proposed of addition reaction of linoleic acid can be seen in Fig 1.
Hydroxylated rubber seed oil has a hydroxyl number 94,903 mgKOH/g and iodine number 40.185 gI2/g. There was an increase for the hydroxyl number and a decrease in iodine number of 40.333 mgKOH/g and 154.052 gI2/g4. Hydroxylation process has improved hydroxyl number amount up to 135.30%.
FTIR spectra of rubber seed oil and hydroxylated rubber seed oil are shown in Fig 2. The -OH groups of hydroxylated rubber seed oil at wave number 3400 cm-1 was appear broader comparing to the spectrum of rubber seed oil. Peak of -CH alkene bond at wave number 3010 cm-1 in the spectrum of rubber seed oil seems clearer with high intensity comparing the spectrum of hydroxylated rubber seed oil. It shows that the hydroxylation process of rubber seed oil has reduced the number of double bonds and those results also supported by reduction of iodine number.
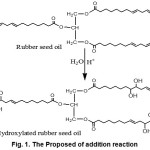 |
Figure 1: The Proposed of addition reaction |
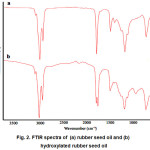 |
Figure 2: FTIR spectra of (a) rubber seed oil and (b) hydroxylated rubber seed oil |
Polyurethane membranepreparation
The preparation of a polyurethane membrane from hydroxylated rubber seed oil and hexamethylene-1,6-diisocyanate through addition reaction can be seen in Fig 3. The visual results of the membrane with HMDI composition is shown in Table 1.
Table 1 shows the visual of polyurethane membrane with composition variation of HMDI.The optimum composition ofhydroxylated rubber seed oil and HMDI was 5: 7 v / w with a polymerization temperature of 90-100°C and a curing temperature of 115-120°C. It showed better results than the other variations. The obtained polyurethane membrane was homogeneous, smooth, dry (not greasy), a little rigid and brownish yellow
Characterization of polyurethane membrane
Filtration processes was done by using mercury-contaminated water. The membrane surface area was 22.051 cm2 with a pressure of 20 bar and a filtration time of 20 minutes. The results of the flux and rejection factors can be seen in Table 2.
Rejection factor was obtained by calculating the concentration of mercury in the feed and permeate using atomic absorption spectrophotometry (AAS). Decoys are used in the form of mercury-contaminated water with a mercury concentration of 0.0703 ppb, resulting permeate contains 0.0008 ppb mercury. So that the resulting rejection factor was 98.86%.
Table 1: Variations in composition in the preparation of polyurethane membrane
|
HRSO1(ml) |
HMDI2(grams) |
Visual description |
|
5 |
4 |
homogeneous, dry, slightly elastic, yellow, slightly bubbly |
|
5 |
5 |
homogenous, slightly bubbly, a little stiff, dry, tawny,, |
|
5 |
6 |
homogeneous, slightly bubbly, stiff, dry, tawny, |
|
5 |
7 |
homogeneous, smooth, slightly stiff, dry, tawny, |
|
5 |
8 |
homogeneous, smooth, stiff, dry, tawny, |
1 HRSO : Hydroxylated rubber seed oil
2 HMDI : Hexamethylene-1,6-diisocyanate
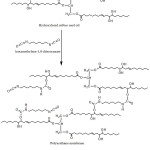 |
Figure 3: Suggested Hydroxylated reaction between linoleic acid and hexamethylene-1,6diisocyanate |
Table 2: Results of flux and rejection factor
|
HRSO(ml) |
HMDI(grams) |
Flux L/m2.h.bar |
rejection factor % |
|
5 |
4 |
0,680 |
* |
|
5 |
5 |
0,612 |
* |
|
5 |
6 |
0,748 |
* |
|
5 |
7 |
0,544 |
98,86 |
|
5 |
8 |
0,680 |
* |
*Not analyzed
The results of the flux and rejection factors show that the polyurethane membrane can be used for mercury filtration from water. The membrane type was reverse osmosis membrane (RO) base on a linear relationship between the pressure applied and the flux.
The FTIR spectrum of polyurethane membran is shown in Fig 4. The spectrum shows that the urethane bond has formed. Urethane bond marked by the wavenumber 3308 cm-1 of N-H, wave number 1373 cm-1 of CN , wave number 1739 cm-1 of -C=O and the absence of -NCO absorption at wave number 2280 cm-1 for spectrum of polyurethane membrane.
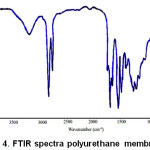 |
Figure 4: FTIR spectra polyurethane membrane |
Polyurethane membrane morphology was observed in cross sectional with magnification 100x and 500x as shown in Fig 5. The cross urethane bond was formed on the membrane. The structure was randomly or amorphous. Irregularities made polyurethane membrane morphology becomes hollow.
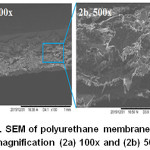 |
Figure 5: SEM of polyurethane membrane with a magnification (2a) 100x and (2b) 500x |
There here two stages of decomposition of the membrane, which has characterized by weight loss at temperature 223⁰C and 386⁰C. The first decomposition was occurred at the weakest point in the structure of macromolecules. The and decomposition happen urethane, bond, the thermostable ware aromatic groups and ester groups of the soft segment in the structure of macromolecules8,9,10,11. Polyurethane membrane weight loss of 50% at a temperature of 410⁰C, weight loss of 90% at a temperature of 472⁰C and residual 4.3% at a temperature of 500⁰C. Thermal analysis using TGA showed two stages of decomposition as shown in Fig 6.
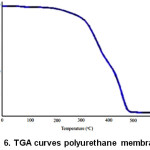 |
Figure 6: TGA curves polyurethane membrane |
Thermal analysis using DTA shows that the value Tg polyurethane membrane synthesized from hydroxylated rubber seed oil was 63°C. As seen in Fig 7,the glass transition was an endothermic shift at baseline because of the heat capacity of the sample was inerease
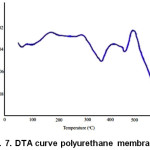 |
Figure 7: DTA curve polyurethane membrane |
Results of mechanical analysis showed that the polyurethane membrane was strong and elastic, with a tensile strength of 1.13 kgf/mm² and elongation 215.36% Fig 8. Polyurethane membrane of rubber seed oil has more hydroxylated crosslinked (hard segment), so that when withdrawn are not prone to elongation, but the effect of the cross-linking of the membrane become stronger. Crosslinking result in higher tensile strength, so that the young modulus was also high.
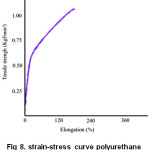 |
Figure 8: strain-stress curve polyurethane membrane |
Conclusion
Hydroxylation process can improve hydroxyl number 135.30% and decrease 73.91% of the iodine number. Increasing hydroxyl number indicates an increase in the -OH group in the rubber seed oil. Hydroxylated rubber seed oil was reacted with hexamethylene-1,6-diisocyanate to produce polyurethane membrane. The optimum composition of the polyurethane membrane synthesized from hydroxylated rubber seed oil and hexamethylene-1,6-diisocyanate was 5:7 (v/w). The obtained membrane had properties of homogeneous, smooth, dry (not greasy), a little stiff, brownish yellow, flux of 0,544 L/m2.h.bar and rejection factor of 98.86%. The results of characterization of polyurethane membrane indicated the formation of a urethane bond at wave number 3308 cm-1, has two stages of decomposition at 223oC and 386oC. The glass transition point 63oC, tensile strength 1,13 kgf/mm2, elongation 215.36%. The obtained polyurethane membrane can be classified on the type of reverse osmosis membrane.
Acknowledgements
This research was supported by the Directorate of Research and Community Service Strengthening the Directorate General Research and development Ministry of Research, Technology and Higher Education (025/SP2H/LT/DRPM/II/2016).
References
- Sarma, S.; Dwi, S.;Hariyadi.J.Tek.Ind. Pert.2011,19, 145-151.
- Dwi, A. S.;Sperisa, D.; Nuryah, D.; Minyana, D. U.Ekui.2010, 9, 11-15.
- Achmad, W.; Iindah, H.; Widayat. Mome.2014, 10, 1-5.
- Salfauqi, N.; Marlina; Saiful; Sitti, S. J.RKL. 2015,10, 188-195.
- Bakare, I. O.;Okieimen,F. E.;Pavithran, C.; Abdul Khalil, H. P. S.;Brahmakumar,M. Mater.and Des.2010, 31, 4274-4280.
CrossRef - Manawer, A.; Deewan, A.; Eram, S.; Fahmina, Z.; Sharif, A. Arab. J. of Chem.2014, 7, 469-479.
CrossRef - Marlina.J. RKL.2007, 6, 67-70.
- Beauty, D.; Uday, K.; Manabendra, M.; Niranjan, K. Ind. Crop.and Prod. 2012, 44, 396-404.
- Jose, H. S. A. J.; Daniel, A. B.; Alvaro, M.; Carlos, A. F.; Franco, D. R. A. J. Mater. Res.2013, 16, 860-866.
CrossRef - Sariah, S.; Luqman, C. A.; Min, M. A.; Mek, Z. S.; Dayang, R. A. B.; Mahiran, B.; Emiliana, R. J.J.Ind. Crops.and Prod.2015, 64, 194-200.
CrossRef - Gurunathan, T.; Smita, M.; Sanjay, K. N.J.Prog.in Org. Coat.2015, 80, 39-48.
CrossRef

This work is licensed under a Creative Commons Attribution 4.0 International License.









