Synthesis of Amorphous Polylactide and Poly(lactide-co-glycolide) Containing High L-form Enantiomer for Use in Controlled Release Drug Delivery
Nuanchai Khotsaeng, Kansiri Pakkethati and Yodthong Baimark
Biodegradable Polymers Research Unit, Department of Chemistry and Center of Excellence for Innovation in Chemistry, Faculty of Science, Mahasarakham University, Mahasarakham 44150, Thailand.
Corresponding Author E-mail: yodthong.b@msu.ac.th
DOI : http://dx.doi.org/10.13005/ojc/320503
In this work, the effect ofL-lactide (LL)copolymerization on the properties of poly(D,L-lactide) (PDLL) and poly(D,L-lactide-co-glycolide) (PDLLG)copolymers and its drug release behaviors were determined and discussed.The copolymers were synthesized by ring-opening polymerization of DLL, LL, and G monomer mixtures. The PDLL with DLL/LL ratios of 100/0-50/50 by mole and the PDLLG with DLL/LL/G ratios of 75/0/25-37.5/37.5/25 by mole were investigated. All the copolymers were completely amorphous.The drug-loadedcopolymer microparticles with a spherical shape and smooth surfacewere prepared by the oil-in-water emulsion solvent evaporation method.Indomethacin was used as a poorly-water soluble model drug.The copolymerization of the LL monomer did not change thein vitro drug release profiles of the PDLL and the PDLLG microparticles significantly.It is suggested that theseamorphous PDLL and PDLLG copolymersthat containhigher L enantiomer amounts have the potential to be developed further as a lower-cost PDLL and PDLLG, respectively,for use as controlled release drug delivery systems.
KEYWORDS:Lactide; Glycolide; Copolymers; Microparticles; Drug delivery
Download this article as:| Copy the following to cite this article: Khotsaeng N, Pakkethati K, Baimark Y. Synthesis of Amorphous Polylactide and Poly(lactide-co-glycolide) Containing High L-form Enantiomer for Use in Controlled Release Drug Delivery. Orient J Chem 2016;32(5). |
| Copy the following to cite this URL: Khotsaeng N, Pakkethati K, Baimark Y. Synthesis of Amorphous Polylactide and Poly(lactide-co-glycolide) Containing High L-form Enantiomer for Use in Controlled Release Drug Delivery. Orient J Chem 2016;32(5). Available from: http://www.orientjchem.org/?p=21754 |
Introduction
Controlled release drug delivery systems made from biodegradable particles provide several benefits over traditional formulations.1Prior to release, the drug is protected from degradation or premature metabolism by the polymeric particle matrix. The release of the drug is sustained over days to months, thereby keeping the drug concentration in the plasma at an effective levelfor longer periods of therapy time and reducingthe toxic side-effectsfrom overdose of the drug. This decreases the frequency of administration and increases patient compliance.2Biodegradable microspheres for drug delivery have been widely made from a variety of biodegradable polyestersdue to their biodegradability and biocompatibility.The removal of these biodegradable polyester-based microspheres at the end of the therapy is not required.Administration of medication via such a system is advantageous because the microspheres can be ingested or injected.3-4
Amorphous poly(D,L-lactide) (PDLL) and poly(D,L-lactide-co-glycolide) (PDLLG) are biodegradable and biocompatible polyesters that have been widely investigated for use as controlled release drug delivery systems.4-6Thisis due to the fact that good drug distribution into the amorphous PDLL and PDLLG matrices can be obtained. The semi-crystalline phases in the poly(L-lactide) (PLL) matrix may induce drug aggregation. A good distribution of the entrapped drug into the microparticle matrices could allow a consistent drug release rate.Controllable molecular weights of the PDLL and the PDLLG are usually synthesized by ring-opening polymerization of DLL and DLL/G monomers, respectively. The higher hydrophilic G units in the PDLLG resulted in a faster biodegradation rate than the PDLL.
An equivalent D-lactic acid/L-lactic acid mixture, called D,L-lactic acid, is used to prepare the DLL monomer.However, L-lactic acid, the monomer precursor of LL, is produced at a larger scale for supplyto food, cosmetic, and pharmaceutical applications. Therefore, the L-lactic acid is easier to find and cheaper than the D,L-lactic acid.The DLL and LL monomers are prepared from the D,L-lactic acid and the L-lactic acid, respectively, by the same procedure. This has the benefit of offering a substantial reduction in cost by the addition of LLto produce lower-cost alternatives to the current commercial PDLL and PDLLG for use in controlled release drug delivery applications.
In the present paper, PDLL and PDLLG copolymers with different LL monomer contents were synthesized by ring-opening polymerization of the DLL/LL and the DLL/LL/G mixtures, respectively. We prepared drug-loaded copolymer microparticles using an oil-in-water emulsion solvent evaporation method. Indomethacin was used as the hydrophobic model drug. The characteristicsof the drug-loaded PDLL and PDLLGmicroparticles containing high LL content andin vitroindomethacin release were determined and compared to the neat PDLL and PDLLG microparticles.
Experimental Section
Materials
Crude D,L-lactide (DLL) and L-lactide (LL) monomers were synthesized fromD,L-lactic acid (85% w/v, 50/50 D-/L-form ratio, Acros Organics) and L-lactic acid (88% w/v, 5/95 D-/L-form ratio, Purac), respectively, by direct polycondensation at 180 °C followed by thermal decomposition at 220 °C under reduced pressure. Crude glycolide (G) monomer was synthesized from glycolic acid (99%, Acros Organics) by the same procedure. The reaction temperatures for the direct polymerization and the thermal decomposition stages were 220 °C and 320 °C, respectively, for preparing the crude G monomer. Crude lactide and glycolide monomers were purified by re-crystallization in ethyl acetate four times. The purified monomers weredried under vacuum at 55°C for 48 h before use in the polymerization.1-dodecanol (98%, Fluka)containing a one-hydroxyl end group was purified by distillation under reduced pressure before use. Stannous octoate [Sn(Oct)2, 95%, Sigma], indomethacin (99.95%, Sigma), and Tween80 (Acros Organics) were used without further purification. All reagents used were analytical grade.
Synthesis of Copolymers
Poly(D,L-lactide)(PDLL) and poly(D,L-lactide-co-glycolide) (PDLLG)copolymers were synthesizedby ring-opening polymerization of the DLL/LL/G mixtures in bulk at 165 °C for 2.5 h under a nitrogen atmosphere. Sn(Oct)2 was used as a catalyst at 0.01 mol% and 1-dodecanol was used as an initiator.Copolymers with a theoretical number-average molecular weight (Mn, theoretical) of 50,000 g/mol were prepared.The 1-dodecanol concentrations of 0.28 and 0.27 mol% were used to synthesize the PDLL and the PDLLG, respectively. The crude copolymers were purified by dissolving in chloroform before precipitating in cool n-hexane. They were then dried to a constant weight in a vacuum at 50 °C for 48 h.
Characterization of Copolymers
The specific optical rotation of the PDLL copolymerswas determined in chloroform at a concentration of 1 g/dL at 25 °C with a Bellingham and StanleyPolarimeter ADP220 at a wavelength of 589 nm. The specific optical rotation of the PDLL was used to calculate the D and L enantiomer contents.7-8
The chemical compositions of the PDLLG copolymers were measured by 1H-NMR spectrometry using a Bruker Advance DPX 300 1H-NMR spectrometer at 25 °C. CDCl3 was used as the solvent, and tetramethysilane was used as the internal standard.
The number-average molecular weight (Mn) and molecular weight distribution (MWD) of the copolymers were determined by Gel Permeation Chromatography (GPC) with a Waters e2695 separations module equipped with PLgel 10 mm mixed B 2 columns operating at 40 °C and employing a refractive index (RI) detector. Tetrahydrofuran was used as the solvent at a flow rate of 1.0 mL/min.
The thermal transition properties of the copolymerswere determined with a Perkin-Elmer Pyris Diamond differential scanning calorimeter (DSC) under a nitrogen flow. For DSC, copolymers of 5 – 10 mg in weight were heated at 10 oC/min over a temperature range of 0 to 200 °C (1st heating scan) to observe their melting temperature (Tm). Then the samples were quenched to 0 °C according to the DSC instrument’s own default cooling mode before heating from 0 to 200 °C (2nd heating scan) to observe their glass transition temperature (Tg). The Tm was measured as the peak value of the endothermal phenomena in the DSC curve. The Tg was taken as the midpoint or half of the heat capacity increment, associated with the glass-to-rubber transition.
Preparation of Drug-Loaded Copolymermicroparticles
The copolymermicroparticlesentrapping the indomethacin model drug were prepared by the oil-in-water emulsion solvent evaporation method. Dichloromethane was used as an organic solvent. 90 mg of copolymer and 10 mg of indomethacin were dissolved in 2.5 mL of dichloromethane (oil phase). The oil phase was slowly added-drop wise into 400 mL of a 2% w/v Tween80 solution in distilled water (water phase) under magnetic stirring. The organic solvents were evaporated in a fume hood for 6 h. The drug-loaded microparticles suspended in the water phase were obtained. The resultingmicroparticles were collected by centrifugation before freeze-drying.
Characterization of Drug-loaded copolymermicroparticles
The morphology ofthe microparticles was observedby scanning electron microscopy (SEM, JEOL JSM-6460LV). The microparticles were sputter-coated with gold to enhance the surface conductivity before scanning. The average size of the microparticles was determined from several SEM images by counting a minimum of 100 particle diameters using the smile view software (version 1.02).
The drug loading of the microparticles was measured by UV-vis spectrophotometry (Lamda 25, Perkin Elmer). For this purpose, the drug-loaded microparticles were completely dissolved in dichlorometane before analysis with a UV-vis spectrophotometer at lmax = 319nm.9The amount of indomethacin was calculated by comparisonwith a standard equation of indomethacin solution in dichloromethane. The standard equation and R2 were y = 0.0181x + 0.0158 and 0.9996, respectively.
The theoretical drug loading content (DLCtheoretical), actual drug loading content (DLCactual), and drug loading efficiency(DLE) were calculated form Equations (1) – (3), respectively. The DLCactual was an average value from three measurements.
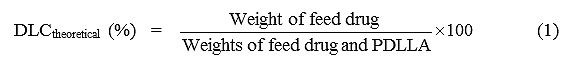
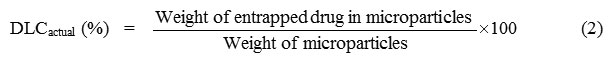
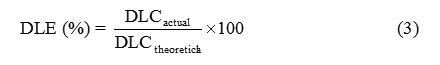
where the weight of the entrapped drug in the microparticles was measured by dissolving the drug-loaded microparticles in dichloromethane before analysis with UV-vis spectrophotometry at lmax = 319 nm.
In Vitro Drug Release Tests
An in vitro drug release test with the microparticles was performed. About 10 mg of drug-loaded microparticles were placed in a pretreated dialysis bag before being incubated in a flask containing 200 mL of 0.02 M phosphate buffer saline (PBS, pH 7.4). The flasks were kept in ashaker incubator at 37 °C and 100 rpm for 48 h. At each desired time, some supernatant was withdrawn and replaced with an equal volume of fresh PBS medium. The release concentration of the indomethacin in the supernatant was determined by a UV-vis spectrophotometer at lmax = 319 nm.9
The amount of indomethacin model drug was calculated by comparisonwith a standard equation of indomethacin solution in PBS.The standard equation and R2 were y = 0.02x + 0.0047 and 0.9994, respectively. The cumulative release percentage of indomethacin (%drug release) was calculated based on the ratio of the drug release at each time and the initial drug content in the microparticles.The drug release profiles were plotted between %drug release and release time. The in vitro drug release tests were performed in triplicate.
Results and Discussion
Characterization of Copolymers
The yields of all the copolymers obtained from the precipitation method were higher than 85%. Table 1 reports the number-average molecular weight (Mn) and molecular weight distributions (MWD) of the copolymers obtained from the GPC curves. All the GPC curves were unimodal. The Mn and MWD values were in the ranges of 47,100-56,400 g/mol and 1.5-2.7, respectively. The Mnvalues obtained from the GPC were close to the values of the theoretical Mn (50,000 g/mol). The Mn of the copolymers was then controlled by the 1-dodecanol concentration.
Table 1: Molecular weight characteristics of copolymers from GPC curves.
|
Comonomer ratio (by mole) |
Mn (g/mol) |
MWD |
| DLL/LL ratio
100/0 90/10 80/20 70/30 60/40 50/50 DLL/LL/G ratio 75/0/25 67.5/7.5/25 60/15/25 52.5/22.5/25 45/30/25 37.5/37.5/25 |
53,200 49,600 53,600 52,100 47,100 49,200 51,500 56,400 53,600 47,600 48,700 51,200 |
2.2 2.7 2.5 2.4 1.6 1.8 1.6 1.5 2.0 1.7 1.9 1.7 |
The PDLL copolymers with different DLL/LL ratios were polymerized from the mixtures of DLL and LL monomers. The feed DLL/LL ratios were 100/0, 90/10, 80/20, 70/30, 60/40, and 50/50 by mole, which corresponded to the feed XD/XLratios of 50/50, 45/55, 40/60, 35/65, 30/70, and 25/75 by mole, respectively, as summarized in Table 2.The final XD/XL ratios of the PDLL copolymers determined from the polarimetryare also reported in Table 2.They were very close to the values of the feed XD/XL ratios. The results suggest that the PDLL copolymers with different XD/XLratios can be synthesized from the DLL/LL mixtures.
The PDLLG copolymers with different DLL/LL/G ratios were synthesized from the mixtures of DLL, LL, and G momomers.The DLL/LL/G ratios were 70/0/25, 62.5/7.5/25, 60/15/25, 52.5/22.5/25, 45/30/25, and 37.5/37.5/25 by mole, as reported in Table 2. The feed DLL-LL/G ratio was constant at 75/25 by mole. The final DLL-LL/G ratios were determined from the 1H-NMR spectrum,an example of which is shown in Figure 1 for the 62.5/7.5/25 DLL/LL/G copolymerincluding peak assignments. It can be seen that the halfDLL and LL units exhibitedthe same peaks as themethine protons (-CH; peak a) and methyl protons (-CH3; peak b) at 5.2 and 1.5 ppm, respectively.10The DLL/LL ratios of the PDLL copolymers could not be determined from the 1H-NMR spectra. The half G units showed peaks of methylene protons (-CH2; peak c) in the range 4.6-5.0 ppm.11The final DLL-LL/G ratios of the PDLLG copolymers calculated from the integral peak areas of the peaks a and c are also reported in Table 2.They were nearly the same values as with the feed DLL-LL/G ratio (75/25 by mole).
 |
Figure 1: 1H-NMR spectrum of PDLLGwith feed DLL/LL/G mole ratio of 67.5/7.5/25 in CDCl3 (peak assignments as shown). |
Table 2: Enantiomer ratios and chemical compositions of copolymers.
|
Comonomer ratio (by mole) |
Feed XD/XL ratio (by mole) a |
Final XD/XL ratio (by mole) b |
Feed DLL-LL/G ratio (by mole) c |
Final DLL-LL/G ratio (by mole)d |
| DLL/LL ratio
100/0 90/10 80/20 70/30 60/40 50/50 DLL/LL/G ratio 75/0/25 67.5/7.5/25 60/15/25 52.5/22.5/25 45/30/25 37.5/37.5/25 |
50/50 45/55 40/60 35/65 30/70 25/75 – – – – – – |
50/50 45/55 41/59 37/63 32/68 27/73 – – – – – – |
– – – – – – 75/25 75/25 75/25 75/25 75/25 75/25 |
– – – – – – 75/25 76/24 78/22 76/24 72/28 72/28 |
a Calculated from feed DLL/LL ratio based on 50/50 D-/L-form ratio and 5/95 D-/L-form ratio for D,L-lactic acid and L-lactic acid, respectively.
b Determined from polarimetry method.
c Calculated from feed DLL/LL/G ratio.
From the 1st heating scan DSC curves, the melting peaks of all the PDLL and the PDLLG copolymers were not found (DSC thermograms not shown). This suggests that the copolymers with the DLL/LL and the DLL/LL/G ratios in the ranges of this study were completely amorphous. It is well known that amorphous polyesters are more appropriate for use in drug delivery. The entrapped drug could be uniformly distributed into the amorphous matrix better than the semi-crystalline matrix. This enhances the consistent drug release.The glass transition temperatures (Tg) of the copolymers obtained from the 2nd heating scan DSC curves were similar and in the ranges of 53-54 °C and 48-49°C for the PDLL and the PDLLG copolymers, respectively. The results indicate that the DLL/LL and DLL/LL/G ratios do not affect the Tgvalues of the copolymers. The Tg values of the PDLLG copolymers were slightly lower than the PDLL copolymers because the Tgof the homopolymersof polyglycolide (37 °C) was lower than the polylactide (55 °C).
Characterization of Copolymermicroparticles
The drug-loaded copolymer microparticles of PDLL and PDLLG were prepared by the oil-in-water emulsion solvent evaporation method. The yields of the microparticles,based on the weights of the feed copolymer and drug,were in the range of 82.4-93.7%.The morphology of the drug-loaded microparticles was observed from the SEM images, as shown in Figures 2 and 3 for the PDLL and the PDLLG, respectively.They were spherical in shape with asmooth surface.The average particle sizes determined from several SEM images are summarized in Table 3. They werein the rangeof 89-113mm.The morphology results suggest that the monomer ratio of the copolymers did not significantly affect theirparticle morphology and size.
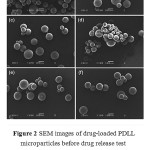 |
Figure 2: SEM images of drug-loaded PDLL microparticles before drug release test prepared from PDLL with feed DLL/LL ratios of (a) 100/0, (b) 90/10, (c) 80/20, (d) 70/30, (e) 60/40, and (d) 50/50 by mole (all scale bars = 100 mm). |
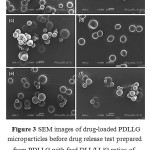 |
Figure 3: SEM images of drug-loaded PDLLG microparticles before drug release test prepared from PDLLG with feed DLL/LL/G ratios of (a) 75/0/25, (b)67.5/7.5/25, (c)60/15/25, (d)52.5/22.5/25, (e)45/30/25, and (f)37.5/37.5/25 by mole (all scale bars = 100 mm). |
The theoretical drug loading content (DLCtheoretical) of all the copolymermicroparticles calculated from Equation (1) was 10 wt%. The actual drug loading content (DLCactual) and the drug loading efficiency (DLE), as summarized in Table 3, were in the ranges of 5.39–6.60% and 53.9–66.0%, respectively. Both DLCactual and DLE did not change significantly with the DLL/LL and the DLL/LL/G ratio.
Table 3: Characteristics of drug-loaded copolymer microparticles.
|
Comonomer ratio (by mole) |
Average particle size (mm) a |
DLCactual (%) b |
DLE (%) c |
| DLL/LL ratio
100/0 90/10 80/20 70/30 60/40 50/50 DLL/LL/G ratio 75/0/25 67.5/7.5/25 60/15/25 52.5/22.5/25 45/30/25 37.5/37.5/25 |
104 ± 34 109 ± 30 111 ± 32 94 ± 23 101 ± 37 113 ± 27 89 ± 16 102 ± 21 93 ± 23 103 ± 13 92 ± 29 92 ± 22 |
5.93 ± 0.04 5.50 ± 0.10 6.08 ± 0.03 5.75 ± 0.34 5.76 ± 0.14 5.39 ± 0.18 5.63 ± 0.02 6.21 ± 0.17 6.09 ± 0.33 6.27 ± 0.18 6.12 ± 0.14 6.60 ± 0.08 |
59.3 55.0 60.8 57.5 57.6 53.9 56.3 62.1 60.9 62.7 61.2 66.0 |
a Determined from several SEM images.
b Calculated from Equation (2).
c Calculated from Equation (3).
In Vitro Drug Release
The in vitro drug release test was performed in PBS at 37 °C for 48 h.The release profiles of indomethacin are illustrated in Figure4 for the PDLL microparticles. All PDLL microparticles with different DLL/LL ratios showed similar sustaineddrug release patterns.An initial burst release of the drug near the particle surfaces was detected within the first 12 h followed bya slower drug release.The initial burst release of the PDLLmicroparticleswas in the range of 30-40%. The drug release was in the range of 60-70% at 48 h of release time.
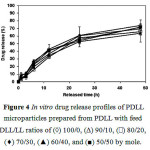 |
Figure 4: In vitro drug release profiles of PDLL microparticles prepared from PDLL with feed DLL/LL ratios of (à) 100/0, (D) 90/10, (ð) 80/20, (¨) 70/30, (▲) 60/40, and (■) 50/50 by mole. |
Figure 5 shows the drug release profiles of the PDLLG microparticles.All PDLLG microparticles with different DLL/LL/G ratios exhibited similar sustained drug release profiles; an initial burst release effect within the first 12 h followed by slower drug release. However, the ranges of theinitial burst release and drug release at 48 h of the PDLLGmicroparticleswere50-60% and 80-90%, respectively. It can be concluded that the DLL/LL and DLL/LL/G ratios did not significantly influence the drug release behaviors of the PDLL and the PDLLG microparticles, respectively. However the PDLLG microparticles exhibited a faster drug release than those of the PDLL microparticles. The drug release results suggest that the PDLL and PDLLG copolymers with different monomer mole ratios showed potential for use as controlled release drug delivery systems.The concentrations of the drug in the plasma could be maintained at the therapeutic level for longer periods of time. Therefore, the frequency of drug administration could be reduced.
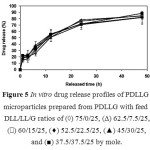 |
Figure 5: In vitro drug release profiles ofPDLLG microparticles prepared from PDLLG with feed DLL/LL/G ratios of (à) 75/0/25, (D)62.5/7.5/25, (ð)60/15/25, (¨)52.5/22.5/25, (▲)45/30/25, and (■)37.5/37.5/25 by mole. |
The predominant drug release mechanism of the PDLL microparticles was proposed to be the drug diffusion process. This was supportedby the SEM image of the PDLL microparticlesin Figure 6(a). The PDLL microparticlesafter 48 h release were still spherical in shape and had a smooth surface.Meanwhile the PDLLG microparticleshad surface erosion, as shown in Figure 6(b). Thus the drug release mechanisms of the PDLLG microparticleswithin 48 h may include both drug diffusion and surface erosion.10This may be due to the higher hydrophilicity and the lower Tgof the PDLLG microparticle matricesthat gave easier water penetration into the particle matrices. Therefore, higher water penetration induces faster surface erosion.
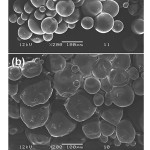 |
Figure 6: SEM images of drug-loaded copolymer microparticles after 48 h release time prepared from (a) 100/0 DLL/LL, and (b) 75/0/25 DLL/LL/G copolymers (all scale bars = 100 mm). |
Conclusions
PDLL and PDLLG copolymers with different DLL/LL and DLL/LL/G mole ratios, respectively, were successfully synthesized via ring-opening polymerization of the mixtures of DLL, LL, and G monomers. They were completely amorphous.The drug-loaded copolymer microparticles of the PDLL and the PDLLGwere prepared by the oil-in-water emulsion solvent evaporation method. These amorphous microparticlescould be used to entrap a poorly-water soluble model drug, indomethacin with a 89-113mm size and a 53.9–66.0%loading efficiency for drug delivery. All the copolymermicroparticles were spherical in shape and had a smooth surface.The copolymerization of the LL units was as high as 50 and 37.5 mol% for the PDLL and the 75/25 PDLLG copolymers, respectively, did not induced PLLA crystallization, and did not affectthe drug release profiles significantly. The PDLLG microparticles showed faster drug release than the PDLL microparticles.
In conclusion, the results presented here show that the amorphous PDLL and PDLLG copolymerssynthesized in this work have the potential to be developed further as drug delivery systems. The amorphous PDLL and PDLLG copolymerswitha high L enantiomer content could provide a viable lower-cost alternative to the commercial PDLL and PDLLG.
Acknowledgements
This work was supported by the Division of Research Facilitation and Dissemination,Mahasarakham University (2015). The Center of Excellence for Innovation in Chemistry (PERCH-CIC), Office of the Higher Education Commission, Ministry of Education, Thailand is also acknowledged. The authors are very grateful to Dr. Jolyon Dodgson, Department of Biology, Faculty of Science, Mahasarakham University for hisimprovement of the English language in this manuscript.
References
- Langer, R., New methods of drug delivery.Science,1990; 249:1527-1533.
- Champion, J.A.; Katare, Y.K.; Mitragotri, S., Particle shape: a new design parameter for micro- and nanoscale drug delivery carriers.J. Controlled Release,2007; 121:3-9.
- Anderson, J.M.; Shive, M.S., Biodegradation and biocompatibility of PLA and PLGA microspheres. Adv. Drug Deliv. Rev.,1997; 28:5-24.
- Freiberg, S.;Zhu, X.X., Polymer microspheres for controlled drug release.Int. J. Pharm.,2004; 282:1-18.
- Liang, R.; Li, X.; Shi, Y.; Wang, A.; Sun, K.; Liu, W.; Li, Y., Effect of water on exenatide acylation in poly(lactide-co-glycolide) microspheres. Int. J. Pharm.,2013; 454:344-353.
- Acharya, A.P.; Lewis, J.S.; Keselowsky, B.G., Combination co-encapsulation of hydrophobic molecules in poly(lactide-co-glycolide) microparticles. Biomaterials, 2013; 34:3422-3430.
- Tsuji, H.;Wada, T.;Sakamoto, Y.;Sugiura, Y., Stereocomplex crystallization and spherulite growth behavior of poly(L-lactide)-b-poly(D-lactide) stereodiblock copolymers. Polymer, 2010; 51:4973-4947.
- Kumar, S.; Bhatnagar, N.; Ghosh, A.K., Effect of enantiomeric monomeric unit ratio on thermal and mechanical properties of poly(lactide). Polymer Bulletin, 2016; 73:2087-2104.
- Srisuwan, Y.; Baimark, Y., Biodegradable poly(D,L-lactide)/lipid blend microparticles prepared by oil-in-water emulsion method for controlled release drug delivery.Orient. J. Chem.,2014; 30:63-69.
- Y Baimark Y.; Srisa-ard, M.,Preparation of Drug-Loaded Microspheres of Linear and Star-Shaped Poly(D,L-lactide)s and Their Drug Release Behaviors, J. Appl. Polym. Sci., 2012; 124:3871-3878.
- Baimark, Y.; Srisa-ard, M.; Puntumchai, A. Synthesis of poly(D,L-lactic acid-co-glycolic acid-co-e-caprolactone) terpolyesters by direct polycondensation, Current Res. Chem., 2010; 2:10-17.

This work is licensed under a Creative Commons Attribution 4.0 International License.









