Research of Polyacrylonitrile Saponification Heterophase Process Mechanism in Different Conditions
Oral K. Beisenbayev, Aziza B. Isa and Anastasya E. Kovaleva
M.Auezov South Kazakhstan state university, Kazakhstan, 160000, Shymkent, Tauke khan avenue, 5.
Corresponding Author Email: isa.aziza@mail.ru
DOI : http://dx.doi.org/10.13005/ojc/310466
Article Received on :
Article Accepted on :
Article Published : 03 Dec 2015
Kinetic regularities of polyacrylonitrile saponification in aqueous, aqueous-alcoholic solutions of alkali and weight were defined. At the same time the environment influence on the kinetics of the process of polyacrylonitrile saponification, the role of diffusion and adsorption saponified agent to the surface of macromolecules as well as the ability to control the depth and degree of saponification hydrophilic product were determined.
KEYWORDS:disperse systems; water-soluble polyelectrolytes; polyacrylonitrile; viscosity; polymer
Download this article as:| Copy the following to cite this article: Beisenbayev O. K, Isa A. B, Kovaleva A. E. Research of Polyacrylonitrile Saponification Heterophase Process Mechanism in Different Conditions. Orient J Chem 2015;31(4). |
| Copy the following to cite this URL: Beisenbayev O. K, Isa A. B, Kovaleva A. E. Research of Polyacrylonitrile Saponification Heterophase Process Mechanism in Different Conditions. Orient J Chem 2015;31(4). Available from: http://www.orientjchem.org/?p=12904 |
Introduction
The rapid development of the petrochemical industry of the Republic of Kazakhstan has led to the need to develop scientific bases of new materials synthesis technology with desired properties based on local raw hydrocarbons, such as water-soluble polyelectrolytes, and their possible application in regulating the properties of disperse systems, particularly stabilizing clay mortar used at drilling process for oil and gas production, as flocculants at wastewater treatment and industrial water when soil structure formation, grouting, shifting sands, binders in the production of concrete, cement, wood chipboard, when creating a quenching medium in metallurgy, etc., is one of the main problems of modern applied Chemistry.
In connection with this a method and technology of acrylic polyelectrolyte synthesis, with the influence of their conformational characteristics responsible for stabilizing and structural actions on various dispersed systems [1—4]. Thus the economic expediency of using polyelectrolytes as regulators – stabilizers in drilling technology is determined not only by their high stabilizing ability but also a number of such performance properties as transportability, stability of the polymers during storage, complete their solubility in water, resistance to salt and temperature aggression ease of handling in all weather conditions and low cost of raw materials. To these requirements most fully soluble polyelectrolytes obtained in the form of dry powders with the desired properties are corresponded.
Method and Results
The purpose of this work is the research of kinetics regularitions of heterophase saponification (hydrolysis) in an aqueous polyacrylonitrile (KO-1) [4], an aqueous-organic media (KO-3) [5] and in the mass (series Uniflok) to develop scientific foundations of powdered water-soluble polymer certain ratio of reactive functional groups. During the work [6, 7] a method of producing the new water-soluble polyelectrolyte based on acrylonitrile (NAK) in powder form was developed. The hydrolysis is carried out in polyacrylonitrile screw mixer under continuous stirring at a temperature of 353K for 1.5 hours, i.e polyacrylonitrile powder treated aliphatic alcohols (ethanol, propanol) at a ratio of 1: 0.6 to 50-60% moisture is then added a concentrated solution of sodium hydroxide in a ratio of PAN to the NaOH -1: 0,5 ÷ 0,7.
Research of heterogeneous kinetics of [7] polyacrylonitrile saponification a process (elemental composition, acid number, saponification degree, a change in viscosity, the ratio of functional groups and IR – spectrum depending on the duration of saponification) makes it possible to determine the mechanism of saponification in different media (water, aqueous alcohol) and weight (Table 1).
Table 1: Characteristics of polymer samples KO-1, KO-3 and “Uniflok” saponification of the duration (0-8 hours)
|
Saponification time. h . |
The content of the residue of nitrogen , % |
The degree of saponification of the polymer (α),% |
Acid number kg ·10-6 КОН/ kg ·10-3 water-soluble polymer |
COONa % |
СОNH2 % |
-NH- % |
η уд/с, |
Solubility in water |
Note |
|
1 |
2 |
3 |
4 |
5 |
6 |
7 |
8 |
9 |
10 |
|
In an aqueous medium (КО-1) |
|||||||||
|
0 0,5 1,0 1,5 2,0 2,5 3,0 |
23,35 12,20 10,0 9,60 9,50 8,85 8,30 |
0,00 47,75 57,17 58,88 59,31 62,09 64,45 |
– – 115 168 398 490 512 |
– – – 21,85 50,14 58,8 60,39 |
– – – – – 33,70 30,36 |
– – – – – 7,5 9,25 |
– – – – – 30,6 29,6 |
insoluble insoluble swells soluble(2/3) soluble -//- -//- |
white powderyellow suspen
red-or susp. red-orange yellow paste creamy paste pale creamy pasta |
|
In aqueous-alcoholic medium (КО-3) |
|||||||||
|
0,5 1,0 1,5 2,0 2,5 3,0 8,0
|
13,20 12,65 11,50 10,50 9,60 9,10 8,20 |
43,46 45,82 50,74 55,03 58,88 61,02 64,88 |
– 105 115 168 466 485 515 |
– 13,15 14,78 21,61 55,15 56,19 60,05 |
– – – – 36,5 32,8 28,9 |
– 16,4 – – 8,5 11,0 11,0 |
– – 13,0 25,0 27,2 27,3 27,0 |
soluble swells soluble (1/3) soluble (2/3) totally totally totally |
yellow suspenred-or susp.
red-brown sus red-yellow sus yellow sus -//- – //- |
|
PAN heterogeneous process saponification in mass pretreated with alcohol to 50-60% moisture content in the mixer CM-1 (Uniflok) |
|||||||||
|
0,5 1,0 1,5 2,0 2,5 3,0 |
12,65 10,40 9,50 9,10 8,20 8,21 |
45,82 55,00 58,15 61,02 64,88 64,90 |
– 175 390 490 495 510 |
– 21,50 50,14 56,19 60,05 60,05 |
– – – 32,8 28,9 28,9 |
– – – 11,0 11,05 11,05 |
– 40,8 42,8 45,1 43,8 43,6 |
Swells soluble soluble -//- -//- -//- |
Red-brown Red-yellow yellow pale cream -//- -//- |
In the process of products saponification, polyacrylonitrile sampling is performed every 15-30 minutes for 7.0 hours. For the start of the reaction was passed upon reaching 353-363K temperature in an aqueous solution (CA-1) 338-343K – water – alcohol medium (KO-3), 353K – in the absence of a liquid dispersion medium, ie. in weight (Uniflok).
At the study of the kinetics saponification heterogeneous process on changing the amount of nitrogen in the reaction mixture revealed a continuous decrease in the nitrogen content in the polymer, accompanied by the release of gaseous ammonia. During 0.5-1.0 hours from the start of the residual nitrogen content in the products of saponification decreases sharply, but in different ways, depending on the reaction conditions from 23.35 to 10.0% – in aqueous (KO-1) and 12.65% – in a hydroalcoholic medium (KO-3) to 10.40% – in bulk, i.e. in the absence of a liquid dispersion medium (Uniflok). Here the degree of saponification in this period increases to 57.17% – in aqueous, to 45.32% – in a hydroalcoholic medium, to 55.0% – in bulk. These data indicate the flow of intensive process of saponification in the first step, which consists in the cyclization of the nitrile groups, and destruction of naphteredine cycles and their conversion into amide and carboxylate groups [8,10,11], as evidenced by IR spectra of the samples show the presence of the corresponding absorption bands 845, 1416, 1570, 1680 • 10-2 m-1 (Figure 1-3). The differences in the kinetics of saponification of the polymer in water and water – alcohol related media, apparently, with the dielectric properties of the medium. Thus, even the reaction of the polymer in the saponification in aqueous – alcoholic medium, according to Barrett [9] indicates the process flow in the surface (diffusion) and the polymeric macromolecule adsorption zones. Therefore, the limiting factors of the reaction are the diffusion speed and saponified adsorption agent (sodium hydroxide) to the surface of the macromolecule. It is known that the dielectric constant of the dispersion medium, which characterizes the ability of a solvent to weaken due and cause the collapse of the solute molecules, to the aquatic environment (E = 81) was higher than for alcohol (E = 8.10). This facilitates almost complete dissociation of sodium hydroxide in an aqueous medium, while in a hydro-alcoholic medium is considerably lower the degree of dissociation, which in turn causes a difference in diffusion constants saponified agent (-OH) in these environments.
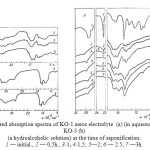 |
Figure 1: Infrared absorption spectra of KO-1 zeros electrolyte (a) (in aqueous solution) and KO-3 (b) (a hydroalcoholic solution) at the time of saponification: 1 — initial., 2 — 0,5h., 3-1,.4-1,5; 5—2; 6 — 2.5; 7 —3h. Click here to View figure |
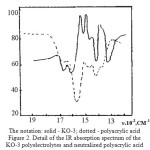 |
Figure 2: The notation: solid – KO-3; dotted – polyacrylic acid Detail of the IR absorption spectrum of the KO-3 polyelectrolytes and neutralized polyacrylic acid Click here to View figure |
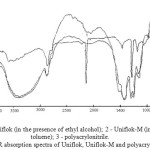 |
Figure 3: The notation: 1 – Uniflok (in the presence of ethyl alcohol); 2 – Uniflok-M (in the presence of toluene); 3 – polyacrylonitrile. IR absorption spectra of Uniflok, Uniflok-M and polyacrylonitrile Click here to View figure |
Increased rate of hydrolysis of polyacrylonitrile mass, apparently due to the fact that although the reaction takes place in the diffusion region, there is no liquid reaction medium that impact on the diffusion process and the adsorption agent is saponified (-OH) groups on the nitrile. Furthermore, the absence of a liquid dispersion medium, apparently increases the transmission rate and hence the amount of heat through the air. Diffused (OH) groups cause chemical reactions of nitrile groups in naftaridine, amide and carboxylate groups.
Polar nature resulting from saponification of the functional groups causes partial saponified surface hydrophilizing polymer dispersions, resulting in a visually swelling the polymer particles in the process of saponification in aqueous medium. With further saponification in the range of 1.0-2.5 hours, the nitrogen content reduced slightly to KO-1 from 10.0 to 8.85%, KO-3 from 9.6 to 12.65%, “Uniflok” from 10.30 to 8.20%, i.e. the saponification degree is increased by an average of 5% to KO-1, 13% to KO-3, 9.8% to “Uniflok”. The process of saponification is on the decline, due, apparently, with the prevalence of the effect of electrostatic repulsion saponified agent (OH) negatively charged carboxylate groups over the effect of disjoining pressure generated by the interaction of the latter with each other. To confirm this, were determined by acid number of the resulting products.
In a period of 1.0-1.5 hours, the acid number increases dramatically, and with further hydrolysis of from 1.5 to 4.0 hours varies slightly (especially in the saponification in the mass – Uniflok), which is associated with a decrease in the rate of saponification in this period as was found earlier by the ammonia release and change in the amount of nitrogen in the products of saponification. The content of cyclic amide and imide groups are also reduced as the number of carboxylate groups. In this sample, hydrolyzed in water and water-alcohol medium has a lower acidity values and the number of carboxylate groups by saponification of equal time, compared with the samples in the saponified mass (Uniflok). Lowering the rate of saponification in this period is due, presumably, increase electrostatic repulsion (-OH) groups due to increased number of negatively charged carboxylate groups. The latter, in addition to the effect of electrostatic repulsion saponified agent (OH) can create steric hindrance for penetration to unsaponified or partially saponified nitrile groups. The time of saponification achieve the degree to 61-62% for each polymer varies respectively for KO-1 (in aqueous medium) – 2.5 hours, KO-3 (in an aqueous alcoholic medium) – 3.0 hours, Uniflok – 2 0 hour. The difference in speeds is apparently due to: in an aqueous medium by electrostatic effect, in an alcoholic medium – the reaction takes place in the diffusion region and OH- ions to penetrate the compact particle is difficult due to the low polarity of the alcohol. High speed saponification weight in case of “Uniflok”, apparently due to the fact that although the reaction takes place in the diffusion region, there is an environment that affects the rate of hydrolyzing, electrostatic factor weakened by concentrated sodium hydroxide solution, hindering of dissociation of carboxylate groups. In this case, the effect of disjoining pressure prevails. The degree of saponification of polymer dispersions at a particular stage correlated ratio of functional groups of varying degrees of saponification, and determining the conformational state of macromolecules. In this regard, the viscosity of the solutions polyelectrolytes – stabilizers KO-1, KO-3 was investigated, which obtained in an aqueous, aqueous-alcoholic medium and in the mass. Samples of the reaction mixture into water (CA-1) and an aqueous-organic (KO-3) media taken at the initial period of reaction up to 1.0 hours will not dissolve in water, which is not possible to measure the viscosity. Samples were obtained by saponification of polyacrylonitrile in heterophase mass dissolve well and the reduced viscosity equals 30-40. Samples taken during 1.5-3.5 hours and the reaction at the beginning, is already dissolved in the aqueous medium thus formed functional groups of different degree of saponification of the polymer surface is hydrophilized to a value causes spontaneous dissolution of the product. The reduced viscosity of the solution increases during the reaction and after 3.5 hours, a constant value slightly reduced by the end of the saponification process. The reduced viscosity is increased in the samples obtained by heterophasic saponification PAN weight in 1.5 hours, and then decreases slightly.
Achieving a particular depth saponification related to the degree of dissociation of the image of the group and the value of the disjoining pressure developed by negatively charged groups, as well as electrostatic effect between the OH groups are saponified agent and the resulting negative saponification infected carboxylate groups.
The last step is defining the mechanism of saponification different nature of the dispersion medium and at a predetermined depth saponification proceeds differently.
Discussion
All this is because the electrostatic effect in the aqueous medium than in an alcohol-water, even more in weight by varying the degree of dissociation of carboxylate groups. Accordingly, in the same sequence and changes in the amount of charge macromolecules polymers obtained in these conditions.
To this effect causes a corresponding change in time and PAN saponification in aqueous, aqueous-organic media and in weight: 3.0; 2.0 and 1.5 hours.
In the study of the absorption spectra of samples of polymers based on acrylonitrile in 4000-600∙10-2m-1 (Figure 1-3) found that the nitrile group, which has the characteristic frequency of the symmetric stretching vibrations, appears as the absorption band at 2250∙10-2m-1 in the spectra of PAN [12,13] and NAC polymer for which the spectra are identical.
Research of the IR-spectra of the polymers in the products of saponification of the organic and aqueous media (Figure 1) and in the absence of a heterophase saponification liquid dispersion medium, i.e. in weight (Figure 3) can be noted that the polymer in the saponification IR NAC polymer undergoes a significant change in the area under study, the rate of hydrolysis and the degree of conversion of nitrile to amide groups depends on the reaction medium (water, alcohol and water in the absence of a liquid dispersion medium), as well as the conditions of the saponification reaction.
However, it can not be detected in the spectra of the absorption band – deformation vibrations in the 1490-1530∙10-2m-1 characterizing the imide rings, as they closed the absorption bands of stretching vibrations of the carboxylate and amide groups, as shown by other authors. For identification of the absorption spectra of the deformation vibrations are fixed differential spectra [14]. Samples were prepared for removal of the spectra in the form of films obtained by drying solutions on water-resistant, transparent to infrared radiation, the crystal plates KRS-6. As a sample for comparison was used partially neutralized polyacrylic acid, obviously not containing imide groups (Figure 2).
The spectra (Figure 3) clearly visible absorption band at 1490-1530∙10-2m-1, which qualifies us as a band -NH- deformation vibrations of imide rings of the polymer powders. the IR spectroscopy incompletely saponified polymer samples NAC in the aqueous solution of sodium hydroxide (CA-1), a hydroalcoholic medium (KO-3) in the absence of a liquid dispersion medium (Uniflok) depending on the duration of saponification showed that saponification takes place in the process two steps (with different velocities depending on the conditions of):
in the first 30 minutes, there is a reduction of the intensity of the absorption band of nitrile groups (2250∙10-2m-1). The process is accompanied by rapid evolution of ammonia, hydration and cyclization nafteridine destruction cycle and formation of an amide group (1680 10-2m-1∙cm-1) [6, 15];
in the next 30-60 minutes the emergence and strengthening of the absorption band in 1408 and 1416∙10-2 m-1, characteristic of the -COOH groups in the macromolecule, show an improvement in the solubility of hydrolyzed polymers, reducing the emission of ammonia and dramatically reduced the rate of hydrolysis (especially in aqueous media KO-1) due to steric factors [16, 17].
Viscosity polyelectrolyte KO-3 prepared in aqueous-alcoholic medium, has a lower value than for polymers KO-1 “Uniflok” obtained in an aqueous medium and weight, which is mainly due to the difference in conformational states of macromolecules. The degree of dissociation of carboxylate groups in the aqueous medium is much higher than in a hydroalcoholic dominated association processes. As a result, the dissociated negatively charged carboxylate groups repel each other in aqueous media and promote the formation of macromolecules unfolded conformation, while the effects of these repulsive reduced in an alcoholic medium and a more compact conformation of macromolecules, which determines the viscosity of solutions of KO-1, KO-3 and “Uniflok”.
The results show that the nature of the dispersion medium plays an important role not only in determining the degree of hydrophilicity and conformational state of macromolecules, but the solubility of the final product. The product obtained in an aqueous medium swells as saponification, is gradually transformed into a gel state, and then a paste form compatible with the thermodynamic stable water solutions in all proportions. Saponification same polymer in an aqueous-alcoholic medium or in the mass occurs swelling of the polymer particles and form a gel, the final product is a yellow powder, soluble in water. This difference of hydrophilicity of the final product of saponification, associated with a greater diffusion, adsorption, depth saponification value of the disjoining pressure of negative functional groups, providing a greater degree of interaction of macromolecules with an aqueous medium. In aqueous-alcoholic medium plays also the role of the precipitant, stay compact form macromolecules and the final product is obtained in powder form.
Saponifying polyacrylonitrile dispersion process takes place in several stages, of which the main ones are:
- saponified diffusion agent (sodium hydroxide) to the surface of macromolecules;
- adsorption on its nitrile group, followed by saponification in nafteridine cycles, imide, amide, carboxylate groups;
- to achieve varying depth saponification related to the degree of dissociation of the formed groups, the size of the disjoining pressure developed by negatively charged groups, as well as electrostatic effect between the OH – groups are saponified agent and formed as a result of saponification negatively charged carboxylate groups.
In this regard, the saponification time varies accordingly 3.0; 2,0; 1.5 hours (in an aqueous, aqueous-alcoholic medium and in mass).
References
- Ahmed K.S, Aripov E.A, Wirski G.M, Glekel F.L, Zaynutdinov S.A. Pogorelsky K.V, Sidorova T. M, Khamraev S.S, Shpilevskaya I.N. Water-soluble polymers and their interaction with the dispersion. Tashkent: Fan, 1969. 250
- Akhmedov K.S, Sataev I.K. Water-soluble polyelectrolytes for drilling. Tashkent: Fan,, 1982.153 .
- Zlotnik D. E., Shvareva Yu.A, Izmailov I.S, Basov T.G. (1971). Chemical processing of drilling and cement solutions.- VNIIBT, vol. 27. M.: Nedra,, p. 152.
- Beisenbayev O.K and other.(1979). The method for producing a reagent for processing clay solutions. The author, the certificate. USSR № 675864 from 29.03.1979.
- Sataev K., Akhmedov K.S Beisenbayev O.K, Kamaryan S.G, Frolov B.(2002). Inventor’s certificate №724523. Publ. №12, from 17.12.2002.
- Beisenbayev O.K and other.(2001). Patent RK 12401 from 08.01.2001. Inventor’s certificate №35065, publ. in №12, from 17.12.2002
- Beisenbayev O.K (2013).Acrylic polyelectrolytes: synthesis, properties and applications. Monograph. – Shymkent: Alem, – 176 p
- Arapov E.A, Saruhanov M.A, Zaynutdinov S.A, Akhmedov K.S (1965).Uzb. chem. Journal ., № 2, p. 52.
- Varretta K.E.J. (1979).The dispersion polymerization in organic media. L.: Chemistry, p. 338
- SchillerA.M., ZuenT.Z. (1956). Ionic derivatives of polyacrylamide. Am Cianamid Co, Stamford Reserche Labjranories, Stamford, Conn. Z.E.C// Industrial und Engineezing Chemestry.– N 48. – P. 21-32.
- Trapeznikov A.A, Borisov B.N Studies of hydrogen bonding in the compositions of the alkyd resin and the polyamide by infrared spectroscopy //Mechanism of film formation processes of polymer solutions and dispersions. – M.: Nauka, – 1966..133-136.
- Gardens F.I, Mikhailov P.V Glazkovsky Y.. Investigation of the process of alkaline saponification of polyacrylonitrile. //Math. Universities series: technology textile industry. – 1965.,4. -.91-97.
- Glazkovsky Yu.V., Mikhailov P.V. The study of the structure of polymer-analogous transformations of polyacrylonitrile products spectroscopy //High-molecular compounds. – 1966.,10.1973-1978.
- Barabanov V.P, Krupin S.V, Zagidullina D.Sh.. Determination of the composition of powdered hydrolyzed polyacrylonitrile // Interuniversity collection. Chemistry and Technology of Organoelement Compounds and polymers. -1977.,6. 55-59.
- Popov V.A, Gladyshev G.P.Heterophase radical polymerization // Usp. – 1973. 42. – 273-300.
- Siffert B., Fernand C., Cotribution al etude du mechanisime a interaction des argiles et des lignosulfonatea – Bull.croupe Frane agriles, 1973..25(2),. 135-148.
- Kurilenko O.D, Prilipko L.G, Mikhalyuk R.V. . On the interaction of polyacrylamide with suspensions // Reports of the Academy of Sciences of the Ukrainian SSR. – 1962.,11. -.1482-1485.

This work is licensed under a Creative Commons Attribution 4.0 International License.









