Preparation and Characterization of Binary Mixture of Efavirenz and Nicotinamide
Erizal Zaini*, Fitri Rachmaini, Fithriani Armin and Lili Fitriani
Department of Pharmaceutical Technology, Faculty of Pharmacy, Andalas University, Padang, West Sumatera, Indonesia Corresponding Author Email erizal.ffua@gmail.com
DOI : http://dx.doi.org/10.13005/ojc/310454
Article Received on :
Article Accepted on :
Article Published : 11 Nov 2015
The purpose of this study was to prepare and characterize the binary mixture of efavirenz and nicotinamide. The binary mixture of efavirenz and nicotinamide (in equimolar ratio) was prepared by solid state grinding and solvent dropped grinding. Characterizations were conducted by powder X-ray diffraction (PXRD), differential thermal analysis (DTA) and scanning electron microscopy (SEM) analysis. Interaction of efavirenz and nicotinamide in liquid states was studied by phase solubility profile. The dissolution rate studies was conducted by using USP type II apparatus in distilled water with 0.5 % sodium lauryl sulfate. Efavirenz dissolved was determined by high performance liquid chromatography (HPLC) with Acetonitrile and acetic acid 1 % as mobile phase. The diffracgram of powder X-Ray analysis showed that both efavirenz and nicotinamide are highly crystalline, and equimolar binary mixtures showed a similar diffraction peaks. Thermal analysis result showed that binary mixture of efavirenz and nicotinamide form a simple eutectic mixture with the eutectic temperature (tE) was 92.7 °C. The SEM analysis depicted that efavirenz and nicotinamide are polyhedral shaped particles, while binary mixture showed a homogenous aggregates of fine needle shaped particles. Phase solubility profile of the binary mixture indicated formation of a soluble complex between efavirenz and nicotinamide in 1:1 molar. The dissolution rate of the binary mixtures were significantly higher compared to the intact efavirenz.
KEYWORDS:binary mixture; eutectic; efavirenz; nicotinamide and dissolution rate
Download this article as:| Copy the following to cite this article: Zaini E, Rachmaini F, Armin F, Fitriani L. Preparation and Characterization of Binary Mixture of Efavirenz and Nicotinamide. Orient J Chem 2015;31(4). |
| Copy the following to cite this URL: Zaini E, Rachmaini F, Armin F, Fitriani L. Preparation and Characterization of Binary Mixture of Efavirenz and Nicotinamide. Orient J Chem 2015;31(4). Available from: http://www.orientjchem.org/?p=12624 |
Introduction
Solid pharmaceutical dosage forms usually contain two or more active pharmaceutical ingredients and excipients; a combination of two or more active ingredients and also with excipients can lead to physical and chemical interactions1. Generally, physical interaction among pharmaceuticals ingredients can be categorized as eutectic mixture (conglomerates), formation of cocrystal (molecular compounds) and solid solution. Eutectic mixture is a mixture of two pharmaceuticals ingredients that has a lower the melting point than the melting point of each component. Cocrystals are the solid compounds that are formed as a result of molecular interaction between molecules of multicomponent in a unique crystal structure. Solid solution is referred to a mixture of two components that miscible in the solid phase2.
Interaction between ingredients in the pharmaceutical dosage forms may cause the formation of new impurities, problems in the manufacturing process, changes in the physicochemical properties of the drug substance (such as stability, solubility, dissolution rate profile, degree of crystallinity and hygroscopicity)3-6. Previous studies reported that interaction between pharmaceutical materials in the binary mixture successfully improve the dissolution rate, such as formation of acyclovir cocrystal with tartaric acid, cocrystal itraconazole with l-malic acid and solid solution of poorly soluble drugs with hydrophilic polymer7-9. In addition simple eutectic mixture between active pharmaceutical ingredients and excipients also could enhance dissolution rate for example binary mixtures of cucurmin with several excipients, binary mixture between fenofibrate-acetylsalicylic acid and flurbiprofen-nicotinamide4,10,11.
Efavirenz is a highly potent non-nucleoside reverse-transcriptase inhibitor used for the treatment of human immunodeficiency virus type-1 infection. It is a very poorly water soluble drugs with aqueous solubility of 0.9 µg/mL and very low intrinsic dissolution rate of 0.037 µg/min/cm. For poorly water soluble drugs, modification of solid states properties of the drugs such as melting point, particle size and crystal form greatly affect the dissolution rate and bioavailability12. Several strategies have been done to improve the dissolution rate of efavirenz such as amorphous state formation, co-micronization with hydrophilic polymer, and preparation of inclusion complexes 12-14. To date, there is no report on preparation of the binary mixture of efavirenz and nicotinamide. Nicotinamide has been used as hydrotropic agent to increase the dissolution rate for many poorly water soluble drugs. It has been widely used for human consumption and classify as GRAS (Generally Recognized as Safe) by FDA15.
The aim of this present work is to prepare and characterize the binary mixture between efavirenz and nicotinamide with the objective to enhance dissolution rate of efavirenz. The physical interaction in liquid and solid states were assessed by using phase solubility studies, X-ray diffraction, differential thermal analysis, scanning electron microscopic and dissolution rate studies.
Material And Methods
Materials
Efavirenz and Nicotinamide were provided from Kimia Farma Ltd. (Indonesia). Ethanol 96% v/v and sodium lauryl sulfate were supplied by Bratachem Ltd. (Indonesia). Methanol, acetonitrile HPLC grade and glacial acetic acid were purchased from Merck (Germany).
Preparation of binary mixture of efavirenz and nicotinamide
Equimolar quantities of binary mixture of efavirenz and nicotinamide were mixed and ground with a mortar and pestle for 1 hours with and without addition of a few drops of ethanol 96% v/v. The resulting binary mixture were further characterize by PXRD, thermal analysis and scanning electron microscopy.
Powder X-ray diffraction analysis
PXRD pattern were measured at room temperature (25°C), with a powder X-ray diffractometer (PAN Analytical, The Netherlands) using Cu Kα radiation. The generator voltage and current were 40 kV and 30 mA, respectively. The 2θ scanning range was from 10 to 40 ° at a step size of 0.02°.
Differential thermal analysis
Thermal behavior of binary mixture was determined by DTA apparatus Mettler-Toledo FP 90 (Switzerland). Accurately weighed samples (5-10 mg) were placed in hermetically sealed aluminum pans. Temperature of scanning was started from 50 to 200 °C at 10 °C/ min.
Scanning electron microscope analysis
Size distribution and crystal habit of the samples were examined using JEOL scanning electron microscope (JEOL model JSM-6360LA, Tokyo, Japan). The samples were mounted on an aluminum stage using adhesive tape and coated with gold. The micrographs were obtained at an excitation voltage of 20 kV and various magnification.
Phase solubility studies
Phase solubility studies were performed based on the Higuchi and Connors method11. Excess of efavirenz was added into conical flasks containing 25 mL of nicotinamide aqueous solution (0.01 – 0.06 M). The flasks were sealed and agitated on an orbital shaker at room temperature for 24 hours at 120 rpm. After equilibration for 24 hours, aliquot was filtered through a membrane filter (0.45 µm), and concentration of efavirenz was determined by high performance liquid chromatography (HPLC). Each experiment was performed triplicate.
Dissolution rate studies
Dissolution rate profile of intact efavirenz and its binary mixture were investigated using a USP paddle dissolution apparatus (Hanson Research SR-08 Plus, USA) at 37 ± 0.5 °C with 900 mL of aqueous solution with 0.5 % of sodium lauryl sulfate which was stirred at 50 rpm. The powder sample equivalent to 100 mg of efavirenz were placed into dissolution medium. At interval time of 5, 15, 30, 45, 60, 80, 100 and 120 minutes, 5 mL of aliquot was withdrawn through a filter and efavirenz dissolved was determined by high performance liquid chromatography (HPLC). Each experiment was performed triplicate.
High performance liquid chromatography analysis
HPLC analysis was conducted with a Shimadzu LC-20AD (Japan) equipped with DAD UV-Vis detector. The HPLC system consisted of a 4.0 x 125 mm LiChrospher RP-18 (Shimadzu, Japan) filled with 5 µm material. Acetonitrile and acetic acid 1 % was used as mobile phase. Efavirenz was detected by UV at wavelength 247 nm. The retention time (tR) of efavirenz was 5.184 min.
Results and Discussion
Mechanical process (such as grinding, compression) and heating could induce the physical and chemical interaction between two solid drugs. Grinding of pyrazinamide and isoniazid (anti-tubercular drugs) can induce the formation of a simple eutectic mixture, whereas eutectic formation of acetaminophen – propyphenazone and acetaminophen – cloperastine hydrochloride through compression has been studied3,5,6. In addition, heating of trimethoprim and sulfamethoxazole also induced a cocrystal phase16. In this study, it was demonstrated that eutectic mixture of efavirenz and nicotinamide can be achieved by solid state grinding and solvent dropped grinding. Grinding process may decrease interparticular spaces and provide more surface area for interaction between efavirenz and nicotinamide.
Possible solid state interaction of the binary mixture of drugs were evaluated by powder X-ray diffraction, DTA thermal and scanning electron microscopic. The powder X-ray diffraction is a reliable technique to identify the solid state interaction between the components. The powder X-ray diffractogram of efavirenz, nicotinamide and its binary mixture are displayed in Figure 1. The diffractogram of efavirenz and nicotinamide showed that these solid phase are highly crystalline powder and has a characteristic diffraction peaks at 2θ = 10.38; 10.90; 12.25; 13.28; 14.2; 16.95; 21.29; 23.12 and 25.03 for efavirenz. While nicotinamide exhibited a characteristic diffraction peak at 2θ = 11.33; 14.80; 19.46; 22.17; 23.20; 25.19; 25.71 and 27.19. The powder X-ray diffractogram of equimolar binary mixtures of efavirenz and nicotinamide after solid state and solvent dropped grinding showed a similar diffraction peaks to its corresponding of the binary mixture. No new diffraction peaks were observed, thereby ruled out formation of solid solution and cocrystal in solid state5 .
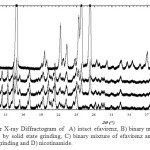 |
Figure 1: Powder X-ray Diffractogram of A) intact efavirenz, B) binary mixture of efavirenz and nicotinamide by solid state grinding, C) binary mixture of efavirenz and nicotinamide by solvent dropped grinding and D) nicotinamide. |
Figure 2. depicted DTA thermogram of efavirenz, nicotinamide and its binary mixture. Efavirenz displayed a sharp endothermic peak at 138.9 °C corresponding to its melting point with heat of fusion about. Nicotinamide showed an endothermic peak at 134.7 °C due to its melting point. The binary mixture of efavirenz and nicotinamide prepared by solid state grinding and solvent dropped grinding gave one sharp endothermic peak at 92.7 °C and 92.9 °C, respectively. A melting endotherm of the binary mixture was lower than the melting points of either efavirenz (138.9 °C) or nicotinamide (134.7 °C). This binary mixture was assumed to form a simple eutectic at 1:1 molar ratio with the eutectic temperature (tE) was 92.7 °C. This results was supported by PXRD analysis.
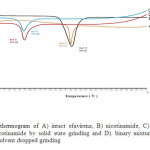 |
Figure 2: DTA thermogram of A) intact efavirenz, B) nicotinamide, C) binary mixture of efavirenz and nicotinamide by solid state grinding and D). binary mixture of efavirenz and nicotinamide by solvent dropped grinding |
To study the particle morphology, scanning electron microscopy analysis was performed for efavirenz, nicotinamide and the binary mixture of the efavirenz and nicotinamide. The scanning electron micrograph of the samples are presented in Figure 3. Efavirenz and nicotinamide are polyhedral shaped particles. The binary mixture of efavirenz-nicotinamide after solid state grinding and dropped grinding showed a homogenous aggregates of fine needle shaped particles.
 |
Figure 3: SEM micrograph of A) intact efavirenz, B) nicotinamide, C) binary mixture of efavirenz and nicotinamide by solid state grinding and D) binary mixture of efavirenz and nicotinamide by solvent dropped grinding. |
The phase solubility of efavirenz as a function of nicotinamide concentration is exhibited in Figure. 4. Solubility of efavirenz increased linearly as a function of nicotinamide concentration in the range 1.10-2 – 6.10-2 M. According to the Higuchi and Connors classification, the phase solubility profile for efavirenz and nicotinamide in aqueous medium is AL type, which indicates the formation of a soluble complex between efavirenz and nicotinamide in equimolar17. The value of stability constant (Ks) of the complex was found to be 13.684 M-1. The low value of Ks indicate that a weak complex was occurred between efavirenz and nicotinamide in aqueous medium18.
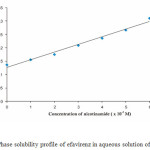 |
Figure 4: Phase solubility profile of efavirenz in aqueous solution of nicotinamide. |
The dissolution profiles of intact efavirenz, the binary mixture of efavirenz-nicotinamide by solid state grinding and by solvent dropped grinding were plotted as shown in Figure 5. The dissolution rate of intact efavirenz was low, which only about 38.86 % of drug dissolved after 80 min in aqueous solution with 0.5 % of sodium lauryl sulfate. This might be attributed to its hydrophobic in nature and low solubility, as shown by floating of the efavirenz particles on the surface of the medium. While the dissolution rate of efavirenz from the binary mixture with nicotinamide by solid state grinding and solvent dropped grinding after 80 min were 55.94 % and 52.75 % respectively. This reveals that these binary mixture showed better dissolution profile than intact efavirenz. The improved dissolution could be attributed to formation of simple eutectic mixture of efavirenz and nicotinamide (1:1 molar). Eutectics have higher free energy due to weak intermolecular interactions between efavirenz and nicotinamide. The enhancement in dissolution rate of active pharmaceutical ingredients through eutectic mixture mechanism also has been demonstrated in binary mixture of cucurmin and several excipients and flurbiprofen-nicotinamide 4,11. Another factor which contributed to improvement of efavirenz dissolution rate was the hydrotropic effect of the nicotinamide in aqueous medium. Nicotinamide was able to destroy water structure and form stacking complex with several drugs on the basis of π electron donor – acceptor and hydrogen bonding 4,5,15.
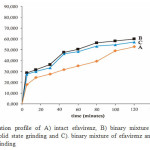 |
Figure 5: Dissolution profile of A) intact efavirenz, B) binary mixture of efavirenz and nicotinamide by solid state grinding and C). binary mixture of efavirenz and nicotinamide by solvent dropped grinding |
Conlusions
The simple eutectic mixtures of efavirenz and nicotinamide were successfully prepared by solid state grinding and solvent dropped grinding. Phase solubility profile indicate formation of soluble complex between efavirenz and nicotinamide 1:1 molar in aqueous medium. Eutectic mixture of these binary mixture have a higher dissolution rate in compared to the intact efavirenz.
References
- Zaini, E.; Sumirtapura, Y.C.; Soewandhi, S. N.; Halim, A.; Uekusa, H.; Fujii, K. Asian J. Pharm. Clin. Res., 2010, 3, 26-29.
- Davis, R.E.; Lorimer, K.A.; Wilkowski, M.A.; Rivers, J.H. ACA Transactions. 2004, 39, 41-61.
- Zalac, S.; Khan, M.Z.; Gabelica, V.; Tudja, M.; Mestrovic, E.; Romih, M., Chem. Pharm. Bull. 1999, 47, 302-307
- Goud, N.R.; Suresh, K.; Sanphui, P.; Nangia, A. Int. J. Pharm. 2012, 439, 63-72.
- Cherukuvada, S.; Nangia, A. Cryst.Eng. Comm. 2012, 14, 2579-2588.
- Sakata, Y.; Tanabe, E.; Sumikawa, T.; Shiraishi, S.; Tokudome, Y.; Otsuka, M. Int. J. Pharm. 2007, 335, 12-19.
- Masuda, T.; Yoshihashi, Y.; Yonemochi, E.; Fujii, K.; Uekusa, H.; Terada, K. Int. J. Pharm, 2012, 422, 160 – 169
- Ober, C.A.; Montgomery, S.E.; Gupta, R.B. Powder Technology, 2013, 236, 122-131
- Chokshi, R.J.; Zia, H.; Sandhu, H.K.; Shah, N.H.; Malick, W.A. Drug Delivery, 2007, 14, 33-45.
- Gorniak, A.; Wojakowska, A.; Karolewicz, B.; Pluta, J. J. Therm. Anal. Calorim. 2011, 104, 1195-1200
- Varma, M.M.; Pandi, J.K. Drug Dev. Ind. Pharm, 2005, 31, 417-423.
- Sathigari, S.; Chadha, G.; Lee, Y. H. P.; Wright, N.; Parsons, D. L.; Rangari, V. K.; Fasina, O.; Babu, R. J. AAPS Pharm. Sci. Tech. 2009, 10, 81-87.
- Costa, M.A.; Seiceira, R.C.; Rodrigues, C.R.; Hoffmeister, C.R.D.; Cabral, L.M.; Rocha, H.V.A. Pharmaceutics. 2013, 5, 1-22.
- Koh, P.T.; Chuah, J. N.; Meghna, T.; Gorajana, A.; Garg. S. Indian J. Pharm. Sci, 2013, 75, 291–301.
- Suzuki H,; Sunada H. Chem. Pharm. Bull., 1998, 46, 125-130
- Zaini, E.; Sumirtapura, Y.C.; Soewandhi, S. N.; Halim, A. Acta Cryst. 2008, A64, C490.
- Higuchi, T.; Connors, K.A. Adv. Anal. Chem. Instr. 1965, 4, 117–212
- Octavia, M. D.; Halim, A.; Zaini, E. J. Chem. Pharm. Res. 2015, 7, 740-747.

This work is licensed under a Creative Commons Attribution 4.0 International License.









