Influence of Reaction Conditions on the Catalytic Oxidation of Cyclohexene with Molecular Oxygen Using a Series of Keggin-Type Polyoxometalate
Ramyah Radman*, Ahmed Aouissi, and Wafa Mekhamer
Chemistry Department, Faculty of science, King Saud University Riyadh, Saudi Arabia
Corresponding Author Email: ramyah8@hotmail.comDOI : http://dx.doi.org/10.13005/ojc/310455
Article Received on :
Article Accepted on :
Article Published : 04 Nov 2015
A series of keggin-type polyoxometalatesnamely; H3PMo12O40,H3PW12O40, Fe1.5PW12O40 and Co1.5PW12O40 were prepared, characterized and tested for the oxidation of cyclohexene by molecular oxygen in acetonitrile medium. The oxidation gives 2-cyclohexen-1-ol, 2-cyclohexen-1-one and cyclohexene oxide,however, 2-cyclohexen-1-one was the major product.TheCo1.5PW12O40catalyst showed the highest catalytic activity for the oxidation reactionand it was chose to study the effect ofvaryingO2 pressure, time, temperature and catalyst weight. The highest percentage of cyclohexen econ version and selectivity of the major product 2-cyclohexen-1-one was obtained at 5bar of O2 pressure, 4h, 70 oC, and 900mg of Co1.5PW12O40 catalyst.
KEYWORDS:Polyoxometalate; cyclohexene; molecular oxygen; catalytic oxidation; Reaction conditions
Download this article as:| Copy the following to cite this article: Radman R, Aouissi A, Mekhamer W. Influence of Reaction Conditions on the Catalytic Oxidation of Cyclohexene with Molecular Oxygen Using a Series of Keggin-Type Polyoxometalate. Orient J Chem 2015;31(4). |
| Copy the following to cite this URL: Radman R, Aouissi A, Mekhamer W. Influence of Reaction Conditions on the Catalytic Oxidation of Cyclohexene with Molecular Oxygen Using a Series of Keggin-Type Polyoxometalate. Orient J Chem 2015;31(4). Available from: http://www.orientjchem.org/?p=12168 |
Introduction
Allylic oxidation of olefins is a very attractive processes for the obtenation of α,β-unsaturated alcohols and ketones which are important transformation due to the presence of a highly reactive carbonyl group.In particular the oxidation products of cyclohexene (CyH) has an important position among the oxidation of olefinic compounds. Cyclohexene is a raw material to produce fine chemicals since its allylic products i.e. 2-cyclohexen-1-ol, 2-cyclohexen-1-one have a great importance in industrial processes and find several applications as intermediates for the manufacture of useful chemicals1-5. 2-cyclohexen-1-ol(enol) is important in natural product synthesis and it can converted to phenol without co-products.2-cyclohexen-1-one (enone) has its main industrial application in the synthesis of medication, pesticides, insect pheromone and adipic acid which used in the production of nylon-6. The epoxidation product of cyclohexene i.e.cyclohexene oxide (epoxy) is also a key intermediate in synthesis of fine chemicals,its importance come from high reactivity due to the opening of the highly strained three- membered ring.Therefore, it is of great interest to develop a more efficient catalyst for the cyclohexene oxidation process.
Several transition metal compounds have been employed as catalysts such as Polyoxometalates (POM). POM are molecular metal-oxygen clusters with discrete structures, they have gained importance due to their distinctive electrochemical, magnetic, medicinal and catalytic properties. Catalysis is the most significant domains of interest due to POMS properties such as oxidative stability, adjustable oxidation states, possible activation of various oxidants and inherent acidity6,7. The oxidation and acid functions of POMS are of great importance in catalysis, because the redox and acidic properties can be controlled at atomic or molecular levels8-10.
The use ofmolecular oxygen O2 as oxidantis considered to be green since no toxic by-products is produced in these reactions. O2is a cheap and green oxidant,it is more readily convenient oxidant for the oxidation reactions but it is relatively unreactive toward the strong bonds of C-H and C=C unless activated by; highly effective catalysts or severe reaction conditions2,11,12.
In this paper the oxidation of cyclohexene using O2 as an oxidizing agent in the presence of POM was studied. The effect of O2 pressure, temperature, time, and catalyst weight on conversion and selectivity were also investigated.
Experimental
Preparation of the catalysts
The heteropolytungstate Co1.5PW12O40 (CoPW), Fe1.5PW12O40 (FePW) were prepared from 12-tugstophosphoric acid H3PW12O40. The H3PW12O40 (HPW) and H3PMo12O40(HPMo) acids was prepared according to the method of Deltcheff et al13. The salt forms were obtained from their counterpart heteropolyacids, as precipitate by adding slowly the required amount of Ba(OH)2.8H2O (to neutralize the three protons) to the aqueous solution of the heteropolyacid, and then the required amount of MSO4.XH2O was added (M=Co,Fe). After eliminating the formed BaSO4 participate, the obtained solution was allowed to stand for few days at 40oC. The salt was recovered from the solution by filtration.
Catalytic Measurement
The reactions were conducted in a stainless steel autoclave equipped with magnetic stirring. The autoclave was thermostated by circulating water which was pumped from a thermostat adjusted at the required temperature. The typical reaction procedure unless otherwise stated was: 10 mL of cyclohexene, 5ml acetonitrile (solvent) and 200 mg of CoPW catalyst were charged into the autoclave. A 5bar pressure of O2 was injected into the autoclave andthe system was stirred and heated at 70 oC for 4h.
The resulting solution was analyzed with a PYE UNICAM gas chromatograph equipped with a flame ionization detector and a capillary column (HP-PLOT Q 30m length and 0.53 internal diameter). The detector and injector temperature was set at 250°C and the total run time was 50min.The identification of the oxidation reactions products was achieved occasionally by Gas Chromatography coupled with a mass selective detector (GC-MS). A Thermo Trace GC Ultra gas chromatograph equipped with AI 3000. For the separation of target compounds, a TR-5 MS-SQC capillary column (30 m length x 0.25 mm internal diameter, phase thickness 0.25 μm) was used with helium as the carrier gas (at a flow rate of 1 ml/min) and the products standard peaks were identified by the device software.From the GC/MS, the oxidation products of cyclohexene (CyH) were two major products 2-cyclohexen-1-one (enone) and 2-cyclohexen-1-ol (enol) and cyclohexene oxide (epoxy) as minor product.
Results and Discussion
Characterization of POM catalysts
Fourier transform infrared (IR) spectra of the samples were recorded with a Shimadzu FTIR Spectrometer 8400S.The IR spectra of the HPW, HPMo, CoPW and FePW catalysts are shown in Fig. 1. The majority of the characteristic bands of the kegging POM structure are found in the finger print region between 500-1200 cm-113,14. The vibration were observed in the following spectra regions:
- The stretching vibration of the central PO4 tetrahedron at ~ 1080 cm-1.
- Stretching vibration involving the central M atom and the terminal O atom (M-Od) ~980cm-1 where (M=W or Mo).
- M-Ob-M bridges (inter bridges between corner sharing octahedral) ~890 cm-1
- M-Oc-M bridges (inter bridges between edge sharing octahedral) i.e. inside a M3O13 set ~(800-790cm-1)
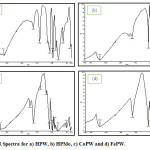 |
Figure 1: IR Spectra for a) HPW, b) HPMo, c) CoPW and d) FePW. Click here to View figure |
The powder X-ray diffraction (XRD) patterns were obtained on Siemens D5000 diffractometer with Cu Kα (l = 0.15418 nm) radiation. The X-ray diffractograms of HPW, HPMo, CoPW and FePW are illustrated in Fig. 2.The results showed the presence of diffraction peaks at 2θ ranging in (7o–10o), (16o–23o), (25o–30o), and (31o–38o). These diffraction characteristic peaks ranges can be assigned to POM having keggin structure15. The diffractograms of HPW and HPMo show that the solids exhibited a degree of crystallinity. The substitution of the protons by cobalt and iron ions increased the intensity of almost all diffraction peaks of acid. The XRD pattern suggests that the 12-tungsto-phosphoric acid secondary structure undergoes structural transformation when the acidic protons are replaced by cobalt and iron cations. This might be due to the fact that the most of the sandwiched water molecules in the form of H3O+ and H5O2+ interacting with Keggin polyanions by hydrogen bonding are lost15-18. This type of interactions is known to make structural transformations that can be detected by powder X-ray diffraction19.
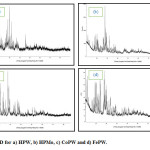 |
Figure 2: XRD for a) HPW, b) HPMo, c) CoPW and d) FePW. Click here to View figure |
Effect of Catalyst Type (Addenda and Counter Ions)
POM generally consist of W and Mo as addenda atoms. Due to the high valence of these elements, POM can act as effective catalysts for the oxidation reaction. The large positive charge of the metal make these compounds capable of accepting electron pairs in vacant d orbitals and form stable M=O complex with O220,21 which make the oxygen atom more electrophilic and therefore readily attacked the reaction site which results in the oxidation to form products. The general reaction pathway is presented in Scheme 1. The allylic oxidation and epoxidation are competitive process in the oxidation of CyH and often occurs simultaneously2,12. Previous studies have revealed that for the catalytic oxidation of CyH the abstraction of the allylic hydrogen to give allylic oxidation products is easier compared to its epoxidation.
For the investigation of the effect of the addenda atoms (Mo and W) on cyclohexene oxidation, the catalytic behaviors of HPMo and HPW heteropolyacids were compared. The results of the conversion and the product distribution are depicted in Fig.3. It can be seen that the catalyst having W as an addenda atom in HPW is more active than the catalyst that has Mo as an addenda atom HPMo. In fact, the conversion obtained over HPW (35%) was higher than that obtained over HPMo (27%). This is because that the peroxotungstate species, generated by the reaction of HPW with O2 is catalytically very active. As for the selectivity the results showed that Mo addenda atoms form more enone (59.2%) than W addenda atoms (51%), the enone formation obtained by HPMo higher than that obtained by HPW is due to the fact that the potential redox of Mo is higher than W one. Whereas, W elements form epoxide (11.6%) while Mo (0%) did not these results suggest that acid character of the W catalyst which is more than Mo leads to the epoxide9,22. For the enol compound it was formed by both Mo and W elements with almost the same selectivity.
The effect of the counter cation (H+, Co2+ and Fe2+) on the cyclohexene oxidation has been achieved by comparing the catalytic behavior of HPW, CoPW and FePW. As shown in Fig.3, it can be seen that the substitution of the protons by the cobalt or by the iron cations, both the conversion and the selectivity for enone and enol (allylic products) were increased in decrement of the epoxide.This was agreed with previous published data12 which usually used catalysts based on cobalt, iron and cupper for allylic oxidation of olefins.
Since the POMs catalyst having Co as a counter cation and W as addenda atoms was found to be the best catalyst of the tested series, CoPW was selected for further investigations.
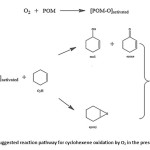 |
Scheme 1: Suggested reaction pathway for cyclohexene oxidation by O2 in the presence of POM. Click here to View scheme |
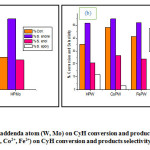 |
Figure 3: a) Effect of the addenda atom (W, Mo) on CyH conversion and products selectivity, b) Effect of the counter cation (H+, Co2+, Fe2+) on CyH conversion and products selectivity. Click here to View figure |
Effect of Catalyst Weight
As seen in Fig. 4 when the weight of the catalyst increased from 200mg – 900mg the conversion increase from 53% to 98%. This suggest that CoPW function as an active sites for the oxidation and with the increase of catalyst amount the number of active sites available for the reaction to progress increase23. SinceCoPW catalyst prefer to form enone (Fig. 3), when the CoPW weight increase a gradual increase in enone and decrease in enolselectivity were also noticed, while for epoxyno significant change in itsselectivity was observed. An experiment without catalyst (blank run) was carried out and no conversion was obtained which indicates that O2 by itself will not aid the oxidation of CyH in the absence of catalyst.
Effect of O2 Pressure
Upon increasing the oxygen pressure P(O2) from 1 – 5 bar the oxidation of CyH improved from 29% to 53% Fig. 4. The presence of excess oxidant favored further oxidation and when P(O2) increase the concentration of the active species(catalyst + oxidant) increase and as a consequence the rate will increase. For the products selectivities Fig. 5, it can seen that the O2/CoPW system prefer to attack the allylic bond in CyH to form enone and enol rather than the C=C bond to form epoxy.There is a slight increase in theselectivity of the major product enone a maximum value were achieved at P(O2) = 5bar while for enol and epoxy a decrease inselectivity was observed due to furtheroxidation to other products.
Effect of Reaction Time
The reaction was carried out at different periods (0.5, 1, 2, 3 and 4h),generally the conversion of CyH increase with time and this is due to the reason that more time is required for the formation of reaction intermediates (substrate +catalyst) which is finally converted to the products. As for the selectivity of the products there is a slight increase with time.The results also shows that the O2/CoPW prefer mostly to attack allylic bond in CyH to form enone and enol and thus limit the C=C epoxidation products i.e. epoxy.
Effect of Reaction Temperature
The conversion of CyH was increased almost lineary with temperature up to 70 oC and a further increase in temperature resulted in the decrease of CyH conversion Fig. 4. Since the CoPW is thermally stable the decrement in conversion at reaction temperature above 70 oC was not due to the catalyst decomposition. The oxidation of CyH well increase with temperature from 60 to 70 oC and this agree with the view point of kinetic. The decrease of conversion after 70 oC may be due to that most reactions with O2 gives a considerable amount of heat and the increase of reaction temperature is unfavorable from the view point of thermodynamic. As for the selectivite is Fig. 5 it remains almost steady for enone and enol but it increased up for epoxy beyond 700C, this may be related to the activation energy of C=C i.e. higher temperature reaction favor reaction with higher activation energy.
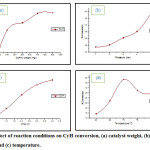 |
Figure 4: Effect of reaction conditions on CyH conversion, (a) catalyst weight, (b) O2pressure, (d) time and (c) temperature. Click here to View figure |
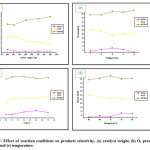 |
Figure 5: Effect of reaction conditions on products selectivity, (a) catalyst weight, (b) O2 pressure, (d) time and (c) temperature. Click here to View figure |
Conclusion
For the oxidation of CyH by O2 in acetonitrile it was found that all these catalysts HPW, HPMo, FePW and CoPW led to enone, enol and epoxy.The results showed that W addenda atoms and Co2+counter cations was the best for the CyH conversion and the enone formation. The effect of various reaction conditions showed that there was a slight change in the products selectivity and enone was the major product in all cases.The best conversion of CyH and selectivity for enone were obtained at P(O2)=5bar, 4h, 70oC, and 900 mg of CoPW catalyst.
References
- Bohstrom, Z.; Rico-Lattes, I.;Holmberg,K. Green Chem. 2010, 12, 1861-1869.
- Fu, Y.;Sun, D.;Qin, M.;Huang, R.;Li, Z. RCS Adv.2012, 2, 3309-3314.
- Yin, C.;Yang, Z.;Li, B.; Zhang, F.;Wang, J. Catal. Lett.2009, 131, 440-443.
- Lee, S. O.;Raja, R.;Harris, K. D. M.;Thomas, J .M.;Johnson, B. F. G.;Sankar, G. Angewandte Chemie Int. Ed.2003, 42,1520-1523.
- Saka, E.T.; Biyiklioglu, Z. J. organometalic chem.2013, 745, 50-56.
- Karcz, R.; Pamin, K.; Plotowicz J.; Haber, J. Catal. Lett.2009, 132, 159-167.
- Li,H.; Yunxiang, Q.; Huan, L.; Bo,F.; ZhenYan, P.; YinYin,Y.; WenWen,Z.; Zhenshan,H. Sci. China Chem.2011, 54, 769-773.
- Kholdeeva,O. A.;Grigoriev,V. A.;Masksimov, G. M.; Zamaraev,K. I. Topics in catalysis.1996, 3, 313-325.
- Duarte, T. A. G.; Santos,I. C. M. S.; Simooes, M. M. Q.; Neves,M. G. P. M. S.; Cavalerio, A. M. V.; Cavalerio,J. A. S. Catal. Lett.2013, 104, 104-111.
- Mizuno, N.; Yamaguchi,K.; Kamata,K. Coordination Chem. Rev.2005, 249, 1944-1956.
- Peng,Y.; Li ,Z.; Tang,R. RCS Adv.2013, 3, 19965-19970.
- Silva,F. P.;Jacinto,M. J.;Landers,R.;Rossi, L. M. Catal. Lett.2011, 141, 432-437.
- Rocchiccioli-Deltcheff,C.; Fournier,M.; Frank, R.; Thouuvenot,R. Inorg. Chem.1983, 22,207-216.
- Rocchiccioli-Deltcheff, C.; Fournier,M.;J. Chem. Soc. Faraday Trans.1991, 87, 3913-3920.
- Aouissi,A.; Al-Deyab, S. S.; Al-Owais, A.;Al-Amro, A.Int. J. Mol. Sci.2010, 11, 2770–2779.
- Fournier,M.; Feumi-Jantou, C.;Rabia,C.;Herve,G.;Launay,S. J. Mater. Chem.1992, 2, 971-978.
- Misono,M.;Chem. Commun. 2001, 13, 1141-1152.
- RAJKUMAR,T.; RANGA RAO, G.;J. Chem. Sci.2008, 120, 587-594.
- Choi,E. Y.; Park,K.; Yang, C. M.; Kim,H.; Son,J. H.; Lee, S. W.;Lee,Y. H.; Min,D.; Kwon,Y. U. Chem. Eur. J.2004, 10, 5535-5540.
- Neumann, R.; Khenkin,M.;Chem. Commun. 2006, 24, 2529-2538.
- Andersson,P. G., Innovative Catalysis in Organic Synthesis: Oxidation, Hydrogenation, and C-X Bond Forming Reactions, First ed.; John Wiley. Germany, (2012)
- lee,K. Y.; Itoh,K.; Hashimoto,M.; Mizuno,N.; Misono,M. New devolpment in selective oxidation 1994, 82, 583-591.
- Pathan, S; Patel,A. R.C.S Catal. Sci. Tech.2014, 3, 648-656.

This work is licensed under a Creative Commons Attribution 4.0 International License.









