Betulinic Acid Glycosides: A Review
Hamisu Abdu, Faujan B H Ahmad and Intan Safinar Ismail
Department of Chemistry, Faculty of Science, University Putra Malaysia, 43400 UPM, Serdang, Selangor, Malaysia.
This paper discusses on the review of most natural and synthesis betulinic acid glycosides. It was revealed that the attachment of sugar substrate at C-3 or C-28 position or both greatly improved the hydrosolubility of betulinic acid and in some cases its pharmacological activities as well.
KEYWORDS:Betulinic acid; glycosides
Download this article as:| Copy the following to cite this article: Abdu H, Ahmad F. B. H, Ismail I. S. Betulinic Acid Glycosides: A Review. Orient J Chem 2012;28(3). |
| Copy the following to cite this URL: Abdu H, Ahmad F. B. H, Ismail I. S. Betulinic Acid Glycosides: A Review. Available from: http://www.orientjchem.org/?p=11869 |
Introduction
Betulinic acid, 3β-hydroxy-lup-20(29)-ene-28-oic acid (1) is a naturally occurring pentacyclic lupane-type triterpene. It shows a broad range of biological and medicinal properties such as inhibition of human immunodeficiency virus (HIV), anti-bacterial, anti-malarial, anti-inflammatory, anthelmintic, anti-oxidant and anti-cancer properties (Yogeeswari and Sriram, 2005). However, the medical applications of betulinic acid in the pharmaceutical industry have been strongly limited since it is insoluble in water (0.02 mg/ml) as well as in organic solvents, causing a difficulty in preparing injectable formulations for biological assays and decreasing its bioavailability in the organism. The introduction of polar groups at C-3 and C-28 positions or both such as phthalates, amino acids or sugar moieties increases, in certain cases, the hydrosolubility and anti-cancer activity (Gauthier et al., 2008; Thibeault et al., 2007). Thus, this paper reviewed most of the betulinic acid glycosides reported in the literatures for over last twenty years. It is hope that work on the betulinic acid glycosides will be of interest to researchers in the field of betulinic acid since this compound is a promising natural product candidate for anticancer and anti HIV drug.
Betulinic acid glycosides in nature
Sung et al. (1990) reported the isolation of three analogous betulinic acid glycosides 4-6 from the leaves of Schefflera octophylla. The plant is used in Vietnamese folk medicine as a tonic drug, antirheumatic agent and treatment of liver diseases. However, no bioactivity studies on the plant was reported.
A year later, Purohit et al. (1991) reported the isolation and characterization of a betulinic acid glycoside from Schefflera venulosa. The extracts of the plant is used in the treatment of liver, rheumatic heart diseases and asthma. Again, no any bioactivity studies was reported on this plant.
In 1993, Orasa et al. reported the isolation of two betulinic acid glycosides 7-8 from the leaves of Schefflera luchanta. To the best of our knowledge these are the first nature betulinic acid glycoside isolated from the plants. The leaves of the plant are widely used in Thai traditional medicine for relieving asthmatic attacks.
Other work by Chatterjee et al. in 1999 resulted in the isolation and structure elucidation of compound 9 .The compound is a conjugated fungal metabolite of betulinic acid and glucose from a resting-cell suspension of Cunninghamella species. The in vitro cytotoxicity of 9 against four human melanoma cell lines namely: MEL-1, MEL-2, MEL-3 and MEL-7 were evaluated. Unfortunately the ED50 values of 9 (> 20 µgmL) showed that the compound is not active against the tested cell-lines. The cytotoxicity of 9 as compared to betulinic acid 1 is shown in Table 1.
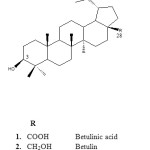 |
Figure 1 Click here to View figure |
Table 1: Compounds 1 and 9 against four human cell lines.
|
Compound |
|
ED50 (µgM/L) |
|
|
|
|
MEL-1 |
MEL-2 |
MEL-3 |
MEL-7 |
|
1 |
1.4 |
1.2 |
1.8 |
4.4 |
|
9 |
> 20 |
> 20 |
> 20 |
> 20 |
MEL-1, MEL-2, MEL-3 and MEL-7 are human melanoma cell lines 1, 2, 3 and 7 respectively.
Work by Adnya et al. in 2000 on the seeds of Combretum quadrangulare finished with the isolation of lupane-type of triterpene glycoside 10, a compound closely related to betulinic acid glycoside. The plant species are widely used in different part of Asia and Africa in folk medicine for the treatment of hepatitis, malaria, respiratory infections and cancer.
Interestingly, the isolation of triterpenoid saponins 11-15 from the roots of pulsatilla Korean was reported by Seong-Cheol et al. in 2005. The compounds were evaluated for their cytotoxic activity against A-549 human carcinoma cell line. Surprisingly, these compounds did not show any cytotoxic activity with ED50 values >10 µg/mL.
Liu et al. in 2006 then reported the isolation of lupane-type triterpenoids glycoside 16 a compound closely related to betulinic acid glycoside, from the leaves of Acanthopanax gracilistylus. The plant is widely used in China as medicine for the treatment of paralysis, arthritis, rheumatism, lameness and liver diseases.
Four years later, in 2009, Nguyen et al. reported the isolation of lupane-triterpene glycoside acankoreoside 17-21 from the leaves of Acanthopanax koreanum. The compounds were evaluated for its cytotoxic activities against cancer cell lines: A 549, HL-60, MCF-7, and U-937. Compounds 18, 20 and 21 showed moderate cytotoxic activity against A-549, HL-60, MCF-7 and U-937 cell-lines with IC50 values ranging from 12.1 to 33.2 and µM, on the other hand compound 17 exhibited considerable cytotoxic activity against A-549 and HL-60 cell lines with IC50 values 8.2µM and 12.1µM respectively. In addition, compound 20 was found to have no activity against HL-60 and U-937 cell-lines. The cytotoxicity activity of compounds 17-21 was shown on Table 2.
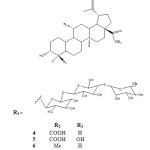 |
Figure 2 Click here to View figure |
Table 2: The effect of lupane-triterpenoid glycosides 17-21 on human cancer cells
| Compound |
IC50 (µM) |
|||
| A-549 | HL-60 | MCF-7 | U-937 | |
| 17 | 8.2 | 12.1 | 28.6 | >100 |
| 18 | 12.1 | 18.9 | 16.9 | 16.5 |
| 19 | 9.2 | >100 | 12.5 | >100 |
| 20 | 32.1 | 33.2 | 16.5 | 21.5 |
| 21 | 21.5 | 22.5 | 16.5 | 18.5 |
A-549: Lung cell line; HL-60: T promyeloctic leukemia; MCF-7: Human breast cancer; U-937: Human macrophage cell line.
Continuation work by Nguyen et al. (2010) reported the isolation of a triterpene glycoside acankoreoside 22 from the leaves of Acanthopanax koreanum. The compound was evaluated for its immune enhancement activity and the results showed that compound 22 generally increased the IFN- and IL-2 release in spleen cells. The structure of the compound 22 was shown on Figure 9 and the effect of the isolated compound 22 on IFN- and IL-2 release was shown on Table 3 and 4 respectively.
 |
Figure 3 |
Table 3: The effect of isolated compound 22 on IFN- release.
| Compound |
IFN- release (pg/mL) |
||
| 5µM | 25µM | 100µM | |
| 22 | 1.25±0.26 | 4.18±0.47 | 2.59±0.08 |
Table 4: The effect of isolated compound 22 on IL-2 release.
| Compound |
IL-2 release (pg/mL) |
||
| 5µM | 25µM | 100µM | |
| 22 | 10.07±2.21 | 12.91±3.47 | 14.76±6.55 |
IFN- and IL-2 are spleen lymphocytes.
Yongxu et al. (2010) reported the isolation and immunological adjuvant activities of betulinic acid glycoside 23 from the root of Pulsatilla chinensis in which the sugar molecule conjugated at both C-3 and C-18 of betulinic acid structure. Compound 23 was found to have slight effects on and an appropriate dose that could be used as a vaccine adjuvant to increase immune responses on mice.
Recently, more complex betulinic acid glycosides were reported by Recently, Akihito et al. (2011). They reported the isolation of new triterpene glycosides 24-25 from the pericarps of stryphnodendron fissuratum. Unfortunately no any bioactivity of these compounds were reported.
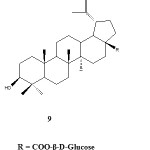 |
Figure 4 Click here to View figure |
Synthesis of betulinic acid glycosides by chemical reactions.
Gauthier et al. (2006) reported on the synthesis of glycosides (β-D-glucosides), α-L-rhamnosides, and α-D-arabinosides 27-29 through the glycosidation of betulinic acid with D-Glucose, L-Rhamnose and D-Arabinose at room temperature in dichloromethane under catalytic promotion of Lewis acid trimethylsilyl trifluoromethane sulfonate (TMSOTF). Subsequent removal of the protecting groups using sodium hydroxide (NaOH, 0.25 N) in CH3OH/THF/H20,( 1:2:1) and regeneration of C-28 acid function of betulinic acid in the presence of tetrakistriphenyl phosphine palladium catalyst and pyrolidine in dry THF gave the above compounds. The compound were tested in vitro for cytotoxicity against three cancerous cell lines A-549, DLD-1, B16-F1 and one healthy (WSI) cell line. The results shows that the cancer cell-lines are 8-12 fold more sensitive to the 3-O-α-L-rhamnopyranoside derivative of betulinic acid 28 with IC50 2.6-3.9 µM than the normal cells(IC50 31µM). The cytotoxity results of compounds 27-29 were shown on Table 5.
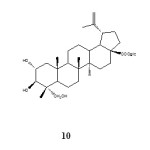 |
Figure 5 Click here to View figure |
Table 5: Cytotoxicity of compounds 27-29
|
Compound |
Cell line IC50 (µM ±SD) |
|||
|
A-549 |
DLD-1 |
B16-F1 |
WSI |
|
|
27 |
>178 |
32±9 |
49±13 |
178 |
|
28 |
2.6±0.6 |
3.9±0.4 |
3.9±0.4 |
31±3 |
|
29 |
10±2 |
17±3 |
11±1 |
475 |
A-549: Human lung carcinoma; DLD-1: Human colorectal adenocarcinoma; B16-F1: Mouse melanoma; WSI: Human normal skin fibroblasts.
Gauthier et al. (2008) reported the synthesis of a naturally occurring betulinic acid saponins 30 bearing α-L-rhamnosyl moiety at C-3 position. Betulinic acid 3β-O– α-L-rhamnosyl isolated from pulsatilla koreanum was easily synthesised through glycosidation process. This compound was synthesized by coupling D-arabinose at the C-3 position of betulinic acid using the following reagents and reaction conditions: tert-butyldiphenylsilyl chloride, immidazole, DMAP, TMSOTF and pyridine refluxed overnight. The last step is incorporating a glucopyranosyl moiety at the C-28 position under phase transfer condition using potassium carbonate (K2CO3) in dichloromethane, water and tetrabutylammonium bromide (Bu4NBr). The results obtained through his procedural leads to the various synthesis of lupane-type saponin derivatives. Compound 30 was shown to exhibit noticeable antiproliferative activity against J774A1 (murine macrophage), WEHI-164 (murine fibro sarcoma) and HEK-293 (human epithelial kidney) cell lines with IC50 0.32-0.79 µM.
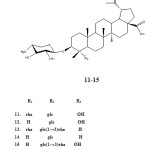 |
Figure 6 Click here to View figure |
Piotr et al. (2008) reported the synthesis of lupane-type saponins bearing mannosyl and their evaluation for anti cancer activity. Glycosylation of 3-O-acetyl betulinic acid in the presence of TMSOTF followed by the debenzolylation with potassium carbonate and subsequent removal of benzoyl protecting groups gave saponin glycosyl-ester 31. The cytotoxic activity of compound 31 is shown on Table 6.
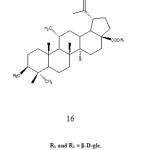 |
Figure 7 Click here to View figure |
Table 6: Cytotoxicity of compound 31 against some cancer cell lines.
| Compound |
Cell line IC50 (µM) |
||
| CEM | MCF-7 | A-549 | |
| 31 | ±50 | ±50 | ±50 |
CEM: T-lymphoblastic leukemia; MCF-7: Human breast carcinoma; A549: Human lung carcinoma.
Dominic et al. (2007) reported the synthesis of 3β-O-monodesmosidic betulinic acids 31-37. Their structure-activity relationship (SAR) study shown in Table 7. It was confirmed that these compounds are generally anti cancer agents in vitro.
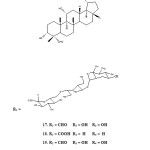 |
Figure 8 Click here to View figure |
Table 7: The cytototoxic activity of compounds 32-37
|
Compound |
Cell line IC50(µmol/L±SD) |
||
|
A-549 |
DLD-1 |
WSI |
|
|
32 |
75 |
329 |
75 |
|
33 |
2.6±0.6 |
3.90±.4 |
31±3 |
|
34 |
10±2 |
17±3 |
47±5 |
|
35 |
±75 |
±75 |
±75 |
|
36 |
34±4 |
15±>1 |
13±3 |
| 37 |
15±2 |
18±2 |
20±1 |
A-549: Human lung carcinoma; DLD-1: Human colorectal adenocarcinoma; WSI: Human normal skin fibroblasts.
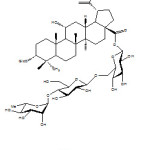 |
Figure 9 Click here to View figure |
Gauthier et al. (2009) reported the synthesis of 28-O-β-D-glucuronide betulinic acid, an acyl glucuronide 38 through glucuronidation using methyl 2,3,4-tri-O-acetyl-1-bromo-D-glucopyranuronate in the presence of K2CO3,Bu4NBr and homogenous solution of dichloromethane and water. Acyl glucuronides has been found to be potential active metabolites of carboxylic acid containing drugs. The in vitro cytotoxicity of 38 was assessed against A549, WS1 and DLD-1 cancer cell line which shows no significant cytotoxic activity on these cells. On the other hand, the in vitro haemolytic activity of 38 was assessed against sheep erythrocytes and the result also revealed that no significant haemolytic activity was observed with HD50 >100µM as shown on Table 8. Interestingly to note that compound 38 can therefore be a good anticancer agent because of its non-cytotoxic, non-haemolytic as well as more water soluble than the corresponding betulinic acid 1.
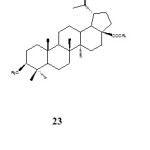 |
Figure 10 Click here to View figure |
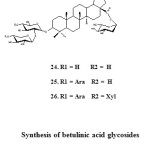 |
Figure 11 Click here to View figure |
Table 8: Cytotoxic and haemolytic activity of compound 71 against human cell lines.
| Cell line | Cytotoxicity IC50 (µM) | Haemolysis HD50 (µM) | ||
| A 549 | DLD-1 | WS1 | ||
| > 100 | > 100 | > 100 |
> 100 |
|
Further work by Gauthier et al. in 2009 reported the synthesis of betulinic acid O-glycosides 39 bearing a chacotriosyl moiety at C-3 position through a step-wise glycosylation. They used 2,3,4,6-tetra-O-benzoyl-α-D-glucopyranosyl trichloroacetamidate in the presence of TMSOTF. The compounds were tested for cytotoxic activity against cancer cell-lines. Betulinic acid 3β-O-chacotriosides were found to be neither cytotoxic nor haemolytic.
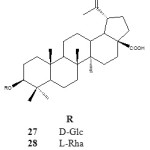 |
Figure 12 Click here to View figure |
Table 9: The cytotoxicity and haemolytic activities of compound 39 against cancer cell lines.
| Compound | HD50(µmolL–1) |
IC50 (µmolL–1) |
||||
| A549 | DLD-1 | MCF-7 | PC-3 | WSI | ||
| 39 | >100 | >50 | >50 | >50 | >50 | >50 |
A-549: Human lung carcinoma; DLD-1: Human colorectal adenocarcinoma; PC-3: Human prostate adenocarcinoma; WSI: Human normal skin fibroblasts.
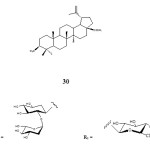 |
Figure 13 Click here to View figure |
Gauthier et al. (2009) reported the synthesis and cytotoxicity of bidesimodic betulinic acid glycoside 40. The compound was tested for cytotoxicity against several cancer cell lines. The compound 40 was found exhibit noticeable cytotoxicity against the cancer cell lines tested. The cytotoxic activity of compound 40 was shown on Table 10.
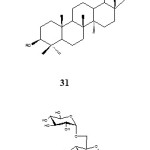 |
Figure 14 Click here to View table |
Table 10: The cytotoxicity of compound 40 against cancer cell line.
| Compound |
Cell lines (IC50 µM) |
||||
| A-549 | DLD-1 | MCF-7 | PC-3 | WSI | |
| 40 | 76±4 | 60±5 | 23±1 | 68±7 | 50±7 |
A-549: Human lung carcinoma; DLD-1: Human colorectal adenocarcinoma; PC-3: Human prostate adenocarcinoma; WSI: Human normal skin fibroblasts.
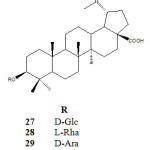 |
Figure 15 Click here to View figure |
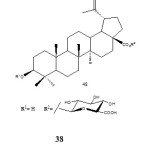 |
Figure 16 Click here to View figure |
In general, saponins are the most relevant triterpene glucoconjugates which are distributed in the plant kingdom. Saponins are known to display antimicrobial effects, protecting plants against mould, hypoglacemic activities, others posses a clear insecticidal activities exhibiting a potent towards a variety of plant pest. The glycosides also constitute a relevant therapeutic application in the treatment of cardiac failure (Purohit et al.1991). They are more water soluble and they are also widely used in the treatment of rheumatism, respiratory infections, arthritis, paralysis and liver disease (Adnya et al. 2000, Liu et al. 2006).
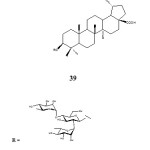 |
Figure 17 Click here to View figure |
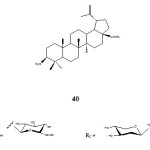 |
Figure 18 Click here to View figure |
Conclusion
It seems that in some cases the bioactivity of betulinic acid can be improved upon the addition of sugar moiety at either C-3 or C-28 or both. Betulinic acid glycosides are more water soluble and easy to formulate, and thus improve its hydrosolubility properties.
References
- Yogeeswari, P. and Sriram, D. Betulinic acid and its derivatives: A review on their biological properties. Current Medicinal Chemistry 12: 657- 666 (2005).
- Gauthier, C., Legault, J., Lavoie, S., Rondeau, S., Tremblay, S. and Pichette, A. (2008). Synthesis of two natural betulinic acid saponins containing α-l-rhamnopyranosyl-(1→2)-α-l-arabinopyranose and their analogues. Tetrahedron 64: 7386–7399.
- Thibeault, D., Gauthier, C., Legault, J., Bouchard, J., Dufour, P. and Pichette, A. Synthesis and structure–activity relationship study of cytotoxic germanicane and lupane-type 3β-O-monodesmosidic saponins starting from betulin. Bioorganic & Medicinal Chemistry 15: 6144-6157 (2007).
- Sung, T. V., Steglich, W. and Adam, G. (1991). Triterpene Glycosides from Schefflera octophylla. Phytochemistry, 30(7): 2349-2356 (1991).
- Purohit, M. C., Pant, G. and Rawat, M. S. W. Abetulinic acid glycoside from Schefflera venulosa. Phytochemistry 30(7): 2419 (1991).
- Orasa, P., Pittaya, T., Water, C. and Kelvin, P. Triterpenoid glycosides from Schefflera luthanta. Phytochemistry, 35(4): 987-992 (1993).
- Chatterjee, P., John, M. P. and Samir, A. K. Glucosidation of Betulinic acid by Cunninghamella species. J. Nat. Prod., 62: 761-763 (1999).
- Adnya, I. K., Yasuhiru, T., Suresh, A., Arjun, H. B., Kim, Q. T. and Shigetoshi, K. Quadranosides VII-XI, Six new Triterpenes Glucosides from the seeds of Combretum quadrangulare. Chem. Pharm. Bull., 48(8): 1114-1120 (2000).
- Seong-Cheol, B. Kim, Y. Lee J. H. and Ahn, B. Z. Triterpenoid Saponins from the Roots of Pulsatilla koreana. J. Nat. Prod., 68: 268-272 (2005).
- Liu, H., Phong, H., Kuo-Hsiung, L. and Chin, Ho. Synthesis and anti-HIV activity of bifunctional betulinic acid derivatives. Bioorganic and Medicinal Chemistry, 14: 2279-2289 (2006).
- Nguyen, X., Phan,V. K., Chau, V. M., Bhui, H. T., Nguyen, H. T., DO, T. H., Kwang, S. S., Jun, H. K., Ji -Young, A., Young-Mi, L., Young, H. K. Structure- activity relationship of lupane triterpene glycosides from Acanthopanax koreanum on spleen lymphocyte IL-2 and IFN-. Biooganic and medicinal chemistry letters, 20: 4927-493 (2010).
- Nguyen, X., Nguyen, H.T., Phan, V. K., Chau, V. M., Yan Ding, Jae-Hyun, Hee-Kuyong, and Young, H. K. Lupane triterpene glycosides from Acanthopanax koreanum and their cytotoxicity activity. Chemical Pharmaceutical Bulletin, 57(9): 986-989 (2009).
- Yongxu, S., Jicheng, L., Haitao, Y. and Chunjie, G. Isolation and evaluation of immunological adjuvant activities of saponins from the roots of pulsatilla chinensis with less adverse reactions. International immunopharmacology, 10: 584-59 (2010).
- Akihito, Y., Sachiko, K., Mitsue, H., Yoshihiro, M. Seven new triterpene glycosides from the pericarps of Stryphnodendron fissuratum. Phytochemistry Letters, 4: 259-266 (2011).
- Gauthier, C. Legault, J., Lebrun, M., Dufour, P., Pichette, A. (2006). Glycosidation of lupane-type triterpenoids as potent in vitro cytotoxic agents. Biorganic med. Chem., 14: 6713-6725 (2006).
- Gauthier, C., Legault, J., Rondeau, S. Trembly, S. and Pichette, A. Synthesis of two natural betulinic acid saponins containing α-L-rhamnopyranosyl-(1→2)-α- L-arabinopyranose and their analogues. Tetrahedron, 64: 7386-7399 (2008).
- Piotr, C., Zbigniew, P., Jana, S. and Miroslav, S. Synthesis of lupane-type saponins bearing mannosyl and 3,6-branched trimannosyl residues and their evaluation as anticancer agents. Carbohydrates Research, 343: 995-1003 (2008).
- Dominic, T., Charles, G., Jean, L., Jimmy, B., Phillipe, D. and Andre´, P. Synthesis and relationship study of cytotoxic germanican and lupine-type 3 β-O- monodesmosidic saponins starting from betulin. Bioorganic and Medicinal Chemistry, 15: 6144-6157 (2007).
- Gauthier, C., Legault, J., Rondeau, S. and Pichette, A. Synthesis of betulinic acid acyl glucuronide for application in anticancer prodrug monotherapy. Tetrahedron Letters, 50: 988-991 (2009).
- Gauthier, C., Jean, L., Marianne, P., Serg, L., Samuel, T., Andre–, P. Synthesis, cytotoxicity and haemolytic activity of chacotriside lupine-type neosaponins and their germicane-type rearrangement products. Biorganic and Med. chem. lett., 19: 2310-2314 (2009).
- Gauthier, C. Legault, J., Serge, L., Simon, R., Samuel, T. and Andre´, P. Synthesis and cytotoxicity of Bidesmosidic betulin and betulinic acid saponins. J. Nat. Prod., 72: 72-81 (2009).

This work is licensed under a Creative Commons Attribution 4.0 International License.









