Ultrasound-Assisted Synthesis and Characterization of 5-Carbonitrile-Functionalized tetrahydropyrimidine Derivatives with Evaluation of Their Antimicrobial Activity
Madhuri J. Suthar1 , Jasmin H. Kumbhani3
, Jasmin H. Kumbhani3 , Keyur D. Bhatt1*
, Keyur D. Bhatt1* , Prakashbhai V. Bishnoi1,2
, Prakashbhai V. Bishnoi1,2 and Parimal Chatrabhuji2
and Parimal Chatrabhuji2
1Faculty of Science, Department of Chemistry, Ganpat University, Kherva, Mehsana-384012, Gujarat, India.
2Pramukh Swami Science and H.D. Patel Arts College Kadi, Mehsana, Gujarat, India.
3Department of Chemistry, M B Patel Science College Anand, S P University, VV Nagar, Gujarat, India.
Corresponding Author E-mail: kdb01@ganpatuniversity.ac.in
DOI : http://dx.doi.org/10.13005/ojc/400134
Article Received on : 03 Nov 2023
Article Accepted on : 06 Jan 2024
Article Published : 17 Jan 2024
Reviewed by: Dr. Debajani Basumatary
Second Review by: Dr. Aiyelabola T. O
Final Approval by: Dr. B. K Sharma
The green synthesis approach employs ultrasound waves as an effective and environmentally friendly strategy to catalyze chemical reactions. Within this framework, carbonitrile-bearing tetrahydropyrimidine derivatives were successfully synthesized. This involved the reaction of malononitrile, urea or thiourea, and variously substituted aldehydes in the presence of morpholine as a catalyst, conducted in aqueous conditions under ultrasonic irradiation. Notably, this method resulted in elevated reaction yields and significantly reduced reaction times when compared to conventional approaches. The synthesized compounds underwent comprehensive characterization using various spectroscopic techniques, including UV-Vis, 1H NMR, 13C NMR, and mass spectrometry. This innovative process aligns with the principles of green chemistry, emphasizing efficiency, sustainability, and the reduction of environmental impact in chemical synthesis.
KEYWORDS:Antimicrobial Activity; Green Synthesis; 4H-Chromene; Ultrasound-Assisted Synthesis
Download this article as:| Copy the following to cite this article: Suthar M. J, Kumbhani J. H, Bhatt K. D, Bishnoi P. V, Chatrabhuji P. Ultrasound-Assisted Synthesis and Characterization of 5-Carbonitrile-Functionalized tetrahydropyrimidine Derivatives with Evaluation of Their Antimicrobial Activity. Orient J Chem 2024;40(1). |
| Copy the following to cite this URL: Suthar M. J, Kumbhani J. H, Bhatt K. D, Bishnoi P. V, Chatrabhuji P. Ultrasound-Assisted Synthesis and Characterization of 5-Carbonitrile-Functionalized tetrahydropyrimidine Derivatives with Evaluation of Their Antimicrobial Activity. Orient J Chem 2024;40(1). Available from: https://bit.ly/423ID66 |
Introduction
The field of organic synthesis has seen significant advancements in recent years, with a focus on developing environmentally friendly and efficient methods to access structurally diverse compounds.1 One such approach is the use of ultrasound-assisted synthesis, a powerful tool that offers several advantages over traditional synthetic techniques.2 Ultrasound-assisted synthesis is characterized by its ability to accelerate chemical reactions through the application of high-frequency sound waves3, leading to enhanced yields, reduced reaction times4, and milder reaction conditions.5
Tetrahydropyrimidine derivatives6 are a class of organic compounds that have garnered significant attention due to their diverse pharmacological properties.7 Their structural versatility makes them promising candidates for drug development8, particularly in the context of antimicrobial agents.9 The carbonitrile-bearing moiety in these compounds often enhances their biological activities, making them an attractive target for synthetic chemistry endeavors.10
The selection of inexpensive, safe, and non-toxic solvents is one of the key components of a green chemical process.11 Water being ample in nature is the primary choice. Additionally, to meet the conditions mentioned earlier12 Development of organic reactions in aqueous environments has been the subject of powerful scientific interest.13
Multicomponent reactions (MCRs) have evolved as efficient chemical processes.14 Clearly, the benefits of the current chemical reaction include a simple, rapid, efficient, and environmental friendly purification approach as well as high product yields.15 “Today, the efficiency of a chemical synthesis may be judged not only by selectivity and total yield16, but also by its raw material, time, human resources17, and energy needs, as well as the toxicity and dangers of the chemicals and the procedures involved.” 18
The problematic issue of creating a modest, environmentally friendly19, and cost-effective reaction technique for medicinal chemistry20 is a important field of both academic and pharmaceutical research.21 Developing MCR processes in aqueous medium is an active field of study in this direction has several benefits, including the absence of carcinogenic effects,22 decreased pollution, lesser cost, and ease of processing, which are beneficial to both the industry and the environment.23
Additionally the synthesis24 of several organic compounds with the aid of ultrasonic technology and the presence of catalysts greatly increase reaction rate25, reduced reaction durations, decreased energy consumption26, increased selectivity, and increased product yield. Compared to traditional procedures, these procedures have revealed to be effective, rapid, clean27, environmentally friendly, and reliable in chemical laboratory.28 One of the emerging areas in organic synthesis, sonochemistry, has significant promise for the development of energy-efficient 29 increase in reaction times brought on by sound waves intense effects (heterogeneous processes)30 and chemical commencement (homogeneous processes).31 Typically, responses encouraged by ultrasonic irradiation are modest to operate32 than those induced by traditional means. Due to our attention in the synthesis of heterocyclic molecules33 with possible biological activity, we were inspired by these discoveries.34
Experimental Section
We acquired the solvents and reagents essential for the synthesis from SDfine Chemicals and Merck Ltd company. Using the open-end capillary device, the melting points (M.P.) of the final derivatives were taken. Mobile phase consisted of a mixture of ethyl acetate:n-hexane (4:6) and TLC plates (TLC silica gel 60 F254) bought from Merck Ltd company. Using a Nicolet 400D spectrometer, the IR spectra were captured in KBr pellets. Using TMS as an internal standard, the 1H and 13C NMR spectra were recorded in DMSO solvent using a Bruker spectrometer functioning at 400 MHz and 100 MHz, separately. The Schmindzu mass spectrophotometer was used to determine the mass spectra data for individual derivative.
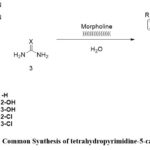 |
Scheme 1: Common Synthesis of tetrahydropyrimidine-5-carbonitrile |
General Procedure
A mixture of substituted aldehyde, malononitrile (1 mmol), and urea or thiourea (1 mmol) in water (10 ml) with catalytic amount of morpholine (0.5 mmol) was irradiated by an ultrasonic irradiation (33 kHz) at room temperature (30˚ C) TLC was used to monitor the reaction’s completion and the mobile phase was ethyl acetate:n-hexane (4:6). The product undergoes a process involving filtration, water washing, drying, and recrystallization using ethanol. The structures of the products were analysed through the FTIR, 1H NMR, 13C NMR spectra and mass spectrometry.
Analytical Discussion
Synthesis of 6-amino-2-oxo-4-phenyl-1,2,3,4-tetrahydropyrimidine-5-carbonitrile (4a)
FTIR (ATR): 3500, 3300, 3020, 3100, 2950, 2850, 1750, 1650, 1350, 1000 cm-1 . Spectra 1HNMR (400 MHz, DMSO-d6 δ ppm): δ=5.6 (s, 1H), 6.5 (s, 2H), 7.61 (s, 1H), 7.29-7.31 (m, 5H), 9.12 (s, 1H) , 13C NMR (100MHz, DMSO-d6, δ, ppm): δ= 50.7, 63.4, 117.3, 126.5, 128.5, 143.5, 150.2, 158.1 MS (m/z): 214.9 (100.0%), m.p.: >200°C; Yield: 97.52 %; Calculated data for C11H10N4O (214.29): C; 61.7 , H; 4.7 , N;26.2%; O, 7.4%. Found data is C; 60.67, H; 4.6 N;25.6%; O, 7.3%
Synthesis of 6-amino-4-(2-hydroxyphenyl)-2-oxo-1,2,3,4-tetrahydropyrimidine-5-carbonitrile (4b)
FTIR (ATR): 3500, 3300, 3050, 3100, 2950, 2750, 1750, 1550, 1350, 1000 cm-1 . Spectra 1HNMR (400 MHz, DMSO-d6 δ ppm): δ=5.6 (s, 1H), 6.5 (s, 2H), 7.61 (s, 1H), 6.83-7.11 (m, 5H), 9.12 (s, 1H) , 13C NMR (100MHz, DMSO-d6, δ, ppm): δ= 50.7, 62.4, 117.3, 125.5, 128.5, 142.5, 150.2, 157.1 MS (m/z): 230.8 (100.0%), m.p.: >200°C; Yield: 97.52 %; Calculated data for C11H10N4O2 (230): C; 57.4, H; 4.4 , N;24.34; O, 13.9%. Found data is C; 56.67, H; 4.4, N;24.6%; O, 12.4%
Synthesis of 6-amino-4-(3-hydroxyphenyl)-2-oxo-1,2,3,4-tetrahydropyrimidine-5-carbonitrile(4c)
FTIR (ATR): 3500, 3200, 3020, 3160, 2950, 2750, 1750, 1550, 1350, 1000 cm-1 . Spectra 1HNMR (400 MHz, DMSO-d6 δ ppm): δ=5.6 (s, 1H), 6.6 (s, 2H), 7.63 (s, 1H), 6.82-7.08 (m, 5H), 9.11 (s, 1H) , 13C NMR (100MHz, DMSO-d6, δ, ppm): δ= 50.7, 63.4, 117.3, 126.5, 128.5, 143.5, 150.2, 158.1 MS (m/z): 230.8 (100.0%), m.p.: >200°C; Yield: 95.52 %; Calculated data for C11H10N4O2 (230): C; 61.7, H; 4.7 , N;26.2%; O, 7.5%. Found data is C; 60.7, H; 4.7, N;25.6%; O, 7.5%
Synthesis of 6-amino-4-(2-chlorophenyl)-2-oxo-1,2,3,4-tetrahydropyrimidine-5-carbonitrile (4d)
FTIR (ATR): 3400, 3250, 3020, 3150, 2950, 2760, 1750, 1500, 1350, 1000 cm-1 . Spectra 1HNMR (400 MHz, DMSO-d6 δ ppm): δ=5.4 (s, 1H), 6.50 (s, 2H), 7.61 (s, 1H), 7.21-7.68 (m, 4H) , 9.12 (s, 1H), 13C NMR (100MHz, DMSO-d6, δ, ppm): δ= 45.6, 50.7, 63.4, 117.3, 126.5, 132.5, 143.5, 150.2, 158.2 MS (m/z): 248.05 (100.0%), m.p.: >200°C; Yield: 96.52 %; Calculated data for C11H9ClN4O (248): C; 53.2 , H; 3.7 , Cl;14.3, N;22.6% , O;6.4%. Found data is C; 50.7, H; 2.7, Cl;13.3, N;21.6%; O, 5.5%
Synthesis of 6-amino-4-(3-chlorophenyl)-2-oxo-1,2,3,4-tetrahydropyrimidine-5-carbonitrile(4e)
FTIR (ATR): 3450, 3200, 3020, 3150, 2850, 2760, 1750, 1400, 1360, 1000 cm-1 . Spectra 1HNMR (400 MHz, DMSO-d6 δ ppm): δ=5.4 (s, 1H), 6.51 (s, 2H), 7.61 (s, 1H), 7.15-7.46 (m, 4H) , 9.12 (s, 1H), 13C NMR (100MHz, DMSO-d6, δ, ppm): δ= 40.6, 50.7, 63.4, 117.3, 126.5, 131.5, 143.5, 150.2, 158.2 MS (m/z): 248.05 (100.0%), m.p.: >200°C; Yield: 96.52 %; Calculated data for C11H9ClN4O (248): C; 53.1 , H; 3.7 , Cl;14.3, N;22.5% , O;6.4%. Found data is C; 50.7, H; 2.7, Cl;13.3, N;21.6%; O, 5.5%
Synthesis of 6-amino-4-phenyl-2-thioxo-1,2,3,4-tetrahydropyrimidine-5-carbonitrile(4f)
FTIR (ATR): 3500, 3230, 3020, 3050, 2830, 2650, 1750, 1400, 1320, 1000 cm-1 . Spectra 1HNMR (400 MHz, DMSO-d6 δ ppm): δ=5.2 (s, 1H), 6.50 (s, 2H), 7.62 (s, 1H), 6.80-7.30 (m, 5H) , 9.12 (s, 1H), 13C NMR (100MHz, DMSO-d6, δ, ppm): δ= 40.6, 50.7, 63.4, 117.3, 126.5, 131.5, 143.5, 150.2, 158.2 MS (m/z): 230.06 (100.0%), m.p.: >200°C; Yield: 92.52 %; Calculated data for C11H10N4S (230.3): C; 57.4 , H; 4.4 , N;24.3% , S;13.9%. Found data is C; 56.7, H; 3.9, N;23.6%; S; 12.4%
Synthesis of 6-amino-4-(2-hydroxyphenyl)-2-thioxo-1,2,3,4-tetrahydropyrimidine-5-carbonitrile (4g)
FTIR (ATR): 3500, 3230, 3020, 3050, 2830, 2650, 1750, 1400, 1320, 1000 cm-1 . Spectra 1HNMR (400 MHz, DMSO-d6 δ ppm): δ=5.1 (s, 1H), 6.48 (s, 2H), 7.60 (s, 1H), 6.83-7.30 (m, 4H) , 9.7 (s, 1H), 9.2 (s, 1H), 13C NMR (100MHz, DMSO-d6, δ, ppm): δ= 42.6, 50.7, 63.4, 117.3, 127.5, 131.5, 143.5, 150.2, 157.2 MS (m/z): 246.1 (100.0%), m.p.: >200°C; Yield: 32.52 %; Calculated data for C11H10N4OS (246.3): C; 57.4 , H; 4.4 , N;22.7% , S;13.9%. Found data is C; 56.7, H; 3.9, S; 12.5%, N;22.6%.
Synthesis of 6-amino-4-(3-hydroxyphenyl)-2-thioxo-1,2,3,4-tetrahydropyrimidine-5-carbonitrile( 4h)
FTIR (ATR): 3400, 3250, 3020, 3030, 2850, 2650, 1750, 1200, 1350, 1000 cm-1 . Spectra 1HNMR (400 MHz, DMSO-d6 δ ppm): δ=5.2 (s, 1H), 6.45 (s, 2H), 7.60 (s, 1H), 7.10-7.35 (m, 4H) , 9.5 (s, 1H), 9.6 (s, 1H), 13C NMR (100MHz, DMSO-d6, δ, ppm): δ= 42.6, 50.7, 63.4, 117.3, 127.5, 131.5, 143.5, 150.2, 157.2 MS (m/z): 246.06 (100.0%), m.p.: >200°C; Yield: 32.5 %; Calculated data for C11H10N4OS (246.3): C; 53.6 , H; 4.1 , N;23.7% , S;13.1%. Found data is C; 52.7, H; 3.9, N;22.7%; S; 12.6%
Synthesis of 6-amino-4-(2-chlorophenyl)-2-thioxo-1,2,3,4-tetrahydropyrimidine-5-carbonitrile (4i)
FTIR (ATR): 3500, 3250, 3050, 3030, 2850, 2650, 1750, 1250, 1350, 1000 cm-1 . Spectra 1HNMR (400 MHz, DMSO-d6 δ ppm): δ=5.3 (s, 1H), 6.43 (s, 2H), 7.60 (s, 1H), 6.83-7.30 (m, 4H) , 9.7 (s, 1H), 13C NMR (100MHz, DMSO-d6, δ, ppm): δ= 42.6, 50.7, 63.4, 117.3, 127.5, 131.5, 143.5, 150.2, 157.2 MS (m/z): 246.06 (100.0%), m.p.: >200°C; Yield: 32.52 %; Calculated data for C11H9ClN4S (264.7): C; 49.9 , H; 3.4 , Cl;13.4, N;21.1 , S;12.1%. Found data is C; 48.7, H; 3.4, Cl;13.3, N;20.7%; S; 12.1%
Synthesis of 6-amino-4-(3-chlorophenyl)-2-thioxo-1,2,3,4-tetrahydropyrimidine-5-carbonitrile (4j)
FTIR (ATR): 3500, 3250, 3050, 3030, 2850, 2650, 1750, 1250, 1350, 1000 cm-1 . Spectra 1HNMR (400 MHz, DMSO-d6 δ ppm): δ=5.3 (s, 1H), 6.45 (s, 2H), 7.6 (s, 1H), 7.11-7.30 (m, 4H) , 9.7 (s, 1H), 13C NMR (100MHz, DMSO-d6, δ, ppm): δ= 42.6, 50.7, 63.4, 117.3, 127.5, 131.5, 143.5, 150.2, 157.2 MS (m/z): 246.1 (100.0%), m.p.: >200°C; Yield: 32.5 %; Calculated data for C11H9ClN4 (264.73): C; 49.9 , H; 3.4 , Cl;13.4, N;21.1 , S;12.1%. Found data is C; 48.7, H; 3.4, Cl;13.3, N;20.7%; S; 12.1%
Result and Discussion
Comparison of solvents
2-Amino-4-aryl-4H-chromene and its derivatives synthesized using urea or thiourea, malononitrile and different aldehyde in 1:1:1 stoichiometric ratio. Morpholine was used as a green catalyst and water as a green solvent. The reaction was carried out under ultrasound irradiation method.
Table 1: Comparison of solvents for the reaction of urea or thiourea, malononitrile 2, and 3-chloro benzaldehyde to afford 6-amino-4-(3-hydroxyphenyl)-2-oxo-1,2,3,4-tetrahydropyrimidine-5-carbonitrile.
|
No. |
Solvent |
Time (minutes) |
Yield % |
|
1 |
No solvent |
07 |
Trace |
|
2 |
Water |
03 |
99% |
|
3 |
Ethanol |
07 |
80% |
|
4 |
Methanol |
06 |
76% |
|
5 |
n-Hexane |
10 |
30% |
|
6 |
Acetone |
10 |
65% |
|
7 |
Propanol |
15 |
68% |
|
8 |
Toluene |
15 |
— |
Comparison of ultrasonic irradiation and conventional methods: When the reaction was carried out using the traditional approach, it gives relatively law yield and took longer to complete, but the reaction carried out under the effect of ultrasonic irradiation gives outstanding product in a fast reaction time. Thus, ultrasonic irradiation was found to be superior over the traditional technique in terms of product yield and efficiency.
Table 2: 6-amino-4-(phenyl derivatives)-2-oxo-1,2,3,4-tetrahydropyrimidine-5-carbonitrile , 6-amino-4-( phenyl derivatives)-2-thioxo-1,2,3,4-tetrahydropyrimidine-5-carbonitrilecompare study under sonication and conventional conditions.
|
No. |
Compound |
– R |
Ultrasonic irradiation |
Conventional method |
||
|
Time (minutes) |
Yield (%) |
Time (minutes) |
Yield (%) |
|||
|
1 |
4a (x=o) |
-H |
2 |
92.40 % |
260 |
80% |
|
2 |
4b(x=o) |
2-OH |
3 |
82.80 % |
260 |
75% |
|
3 |
4c(x=o) |
3-OH |
2 |
71.82 % |
260 |
65% |
|
4 |
4d(x=o) |
2-Cl |
3 |
83.12 % |
260 |
75% |
|
5 |
4e(x=o) |
3-Cl |
3 |
98.00 % |
260 |
85% |
|
6 |
4f (x=s) |
-H |
2 |
96.52 % |
260 |
80% |
|
7 |
4g(x=s) |
2-OH |
3 |
90.41 % |
260 |
75% |
|
8 |
4h(x=s) |
3-OH |
3 |
76.72 % |
260 |
70% |
|
9 |
4i(x=s) |
2-Cl |
3 |
72.91 % |
260 |
65% |
|
10 |
4j(x=s) |
3-Cl |
3 |
97.00% |
260 |
85% |
Table 3: Effect of amount of catalyst in the synthesis of the product 4e
|
No. |
Amount of morpholine (equivalent %) |
Time (minutes) |
Yield % |
|
1 |
Trace |
02 |
Trace |
|
2 |
5 |
03 |
98 |
|
3 |
10 |
04 |
96 |
|
4 |
15 |
06 |
92 |
|
5 |
20 |
05 |
92 |
|
6 |
25 |
05 |
90 |
|
7 |
30 |
05 |
87 |
Table 4: Effect of Time in the synthesis of the product 4e
|
No. |
Solvent |
Time (minutes) |
Yield % |
|
1 |
Water |
03 |
98 |
|
2 |
Water |
05 |
96 |
|
3 |
Water |
10 |
97 |
|
4 |
Water |
15 |
97 |
|
5 |
Water |
20 |
97 |
|
6 |
Water |
25 |
97 |
Table 5: Effect of Temperature in the synthesis of the product 4e
|
No. |
Solvent |
Temperature (C˚ ) |
Time (minutes) |
Yield % |
|
1 |
Water |
20 |
5 |
82 |
|
2 |
Water |
25 |
5 |
82 |
|
3 |
Water |
30 |
5 |
85 |
|
4 |
Water |
35 |
3 |
98 |
|
5 |
Water |
40 |
5 |
80 |
|
6 |
Water |
45 |
5 |
80 |
|
7 |
Water |
50 |
5 |
80 |
Antibacterial Activity of the given samples against Staphylococcus aureus
Staphylococcus aureus MTCC 7443 strain was used in the study. In Mueller Hinton, an agar well diffusion method was used to assess the mentioned microbial isolate for antibacterial susceptibility. S. aureus was injected into nutrient broth on agar (MHA) plates, and it was incubated at 37°C for the whole night. Bacterial culture broth was used to make culture MHA plates. Various samples in a concentration of 40–50 mg/ml were placed in dimethyl sulfoxide (DMSO). Using a sterile cork-borer as support, 6 mm wells were drilled into the inoculation medium. 50 μl of the provided samples and a positive control (gentamicin 20 mg/ml) were added to each well. It was incubate at 37°C for an entire night and to diffuse for about 30 minutes at room temperature. Following incubation, plates were examined to see if a clear zone had developed around the wells. A measurement of the zone of inhibition (ZOI) in millimetres was made.
Table 6: Diameter of zones of inhibition (mm) of given samples against S. aureus at 40-50 mg/ml concentration.
|
Sr.No |
Compound No. |
-R |
Concentration (mg/ml) |
Zone of Inhibition (mm) |
|
1 |
4a (x=o) |
-H |
50 |
8 |
|
2 |
4b(x=o) |
2-OH |
50 |
9 |
|
3 |
4c(x=o) |
3-OH |
50 |
13 |
|
4 |
4d(x=o) |
2-Cl |
50 |
10 |
|
5 |
4e(x=o) |
3-Cl |
50 |
15 |
|
6 |
4f (x=s) |
-H |
40 |
14 |
|
7 |
4g(x=s) |
2-OH |
50 |
13 |
|
8 |
4h(x=s) |
3-OH |
50 |
12 |
|
9 |
4i(x=s) |
2-Cl |
40 |
10 |
|
10 |
4j(x=s) |
3-Cl |
50 |
17 |
|
|
S. aureus |
|
20 (gentamicin) |
40 |
 |
Figure 1: Chart Title |
Conclusion
Using water as a sustainable solvent, we have developed an ecologically friendly way to synthesise derivatives of 6-amino-2-oxo-4-phenyl-1,2,3,4-tetrahydropyrimidine-5-carbonitrile in a one-pot multicomponent reaction under ultrasonic irradiation. This method presents several advantages, including enhanced product yields, reduced reaction times, and a straightforward setup in compare to conventional method. In this work, we observe a comparison between eco-friendly and traditional procedures. Notably, the eco-friendly approach leads to a significant boost in production efficiency. The study involves optimization studies, variations in solvents, reaction time, temperature and the quantity of the base. Ultimately, our research suggests that employing water as the solvent in this process is the most efficient means of achieving optimal results. Additionally, a noteworthy point is that many of the compounds is showing promising antimicrobial activities.
Acknowledgements
The authors would like to express their gratitude to the Ganpat university, mehsana-gozaria highway.
Conflict of interest
The authors claim that they have no known financial conflicts of interest or close personal relationships that would appear to have impacted the research provided in this study.
References
- Wan, J.-P.; Liu, Y. Recent advances in new multicomponent synthesis of structurally diversified 1, 4-dihydropyridines. RSC advances 2012, 2 (26), 9763-9777.
CrossRef - Banerjee, B. Recent developments on ultrasound-assisted one-pot multicomponent synthesis of biologically relevant heterocycles. Ultrasonics sonochemistry 2017, 35, 15-35.
CrossRef - Puri, S.; Kaur, B.; Parmar, A.; Kumar, H. Applications of ultrasound in organic synthesis-a green approach. Current Organic Chemistry 2013, 17 (16), 1790-1828.
CrossRef - Prasanna, B. L.; Rao, B. S.; Muralidhar, P.; Nagaraju, K.; Maddila, S. A swift, facile and multicomponent synthesis of 4H-pyran-3-carbonitrile analogues via grinding process under solvent-free conditions. Chemical Data Collections 2022, 38, 100836.
CrossRef - Mohammadi Ziarani, G.; Kheilkordi, Z.; Gholamzadeh, P. Ultrasound-assisted synthesis of heterocyclic compounds. Molecular Diversity 2020, 24, 771-820.
CrossRef - Alharthi, A. A.; Alotaibi, M.; Shalwi, M. N.; Qahtan, T. F.; Ali, I.; Alshehri, F.; Bakht, M. A. Photocatalytic-driven three-component synthesis of 1, 2, 3, 4-tetrahydropyrimidine-5-carbonitrile derivatives: A comparative study of organocatalysts and photocatalysts. Journal of Photochemistry and Photobiology A: Chemistry 2023, 436, 114358.
CrossRef - Costa, R. F.; Turones, L. C.; Cavalcante, K. V. N.; Rosa Júnior, I. A.; Xavier, C. H.; Rosseto, L. P.; Napolitano, H. B.; Castro, P. F. d. S.; Neto, M. L. F.; Galvão, G. M. Heterocyclic compounds: pharmacology of pyrazole analogs from rational structural considerations. Frontiers in Pharmacology 2021, 12, 666725.
CrossRef - Li, J. J. Heterocyclic chemistry in drug discovery; John Wiley & Sons, 2013.
- Asif, M.; Imran, M. Antimicrobial activities of various thiazine based heterocyclic compounds: a mini-review. Mini-Reviews in Organic Chemistry 2022, 19 (2), 166-172.
CrossRef - Varma, R. S. Solvent‐free synthesis of heterocyclic compounds using microwaves. Journal of Heterocyclic Chemistry 1999, 36 (6), 1565-1571.
CrossRef - Płotka-Wasylka, J.; Rutkowska, M.; Owczarek, K.; Tobiszewski, M.; Namieśnik, J. Extraction with environmentally friendly solvents. TrAC Trends in Analytical Chemistry 2017, 91, 12-25.
CrossRef - Cravotto, G.; Borretto, E.; Oliverio, M.; Procopio, A.; Penoni, A. Organic reactions in water or biphasic aqueous systems under sonochemical conditions. A review on catalytic effects. Catalysis Communications 2015, 63, 2-9.
CrossRef - Nogueira, T. C. M.; de Souza, M. V. Green Synthesis of Five-and Six-membered N-heterocycles by Ultrasonic Irradiation in Aqueous Media. Current Green Chemistry 2021, 8 (2), 99-126.
CrossRef - Ruijter, E.; Orru, R. V. Multicomponent reactions–opportunities for the pharmaceutical industry. Drug Discovery Today: Technologies 2013, 10 (1), e15-e20.
CrossRef - Xin, X.; Wang, Y.; Kumar, S.; Liu, X.; Lin, Y.; Dong, D. Efficient one-pot synthesis of substituted pyridines through multicomponent reaction. Organic & Biomolecular Chemistry 2010, 8 (13), 3078-3082.
CrossRef - Toure, B. B.; Hall, D. G. Natural product synthesis using multicomponent reaction strategies. Chemical Reviews 2009, 109 (9), 4439-4486.
CrossRef - Younus, H. A.; Al-Rashida, M.; Hameed, A.; Uroos, M.; Salar, U.; Rana, S.; Khan, K. M. Multicomponent reactions (MCR) in medicinal chemistry: a patent review (2010-2020). Expert Opinion on Therapeutic Patents 2021, 31 (3), 267-289.
CrossRef - Ghasemzadeh, M. A.; Mirhosseini-Eshkevari, B.; Tavakoli, M.; Zamani, F. Metal–organic frameworks: advanced tools for multicomponent reactions. Green Chemistry 2020, 22 (21), 7265-7300.
CrossRef - Mani, S.; Raju, R.; Raghunathan, R.; Arumugam, N.; Almansour, A. I.; Kumar, R. S.; Perumal, K. Environmentally friendly domino multicomponent strategy for the synthesis of pyrroloquinolinone hybrid heterocycles. RSC advances 2022, 12 (24), 15440-15446.
CrossRef - Toenjes, S. T.; Gustafson, J. L. Atropisomerism in medicinal chemistry: challenges and opportunities. Future medicinal chemistry 2018, 10 (4), 409-422.
CrossRef - Zhao, X.; Modur, V.; Carayannopoulos, L. N.; Laterza, O. F. Biomarkers in pharmaceutical research. Clinical chemistry 2015, 61 (11), 1343-1353.
CrossRef - Candeias, N. R.; Cal, P. M.; Andre, V.; Duarte, M. T.; Veiros, L. F.; Gois, P. M. Water as the reaction medium for multicomponent reactions based on boronic acids. Tetrahedron 2010, 66 (14), 2736-2745.
CrossRef - Verma, C.; Haque, J.; Quraishi, M.; Ebenso, E. E. Aqueous phase environmental friendly organic corrosion inhibitors derived from one step multicomponent reactions: a review. Journal of Molecular Liquids 2019, 275, 18-40.
CrossRef - Mamidala, S.; Aravilli, R. K.; Vaarla, K.; Vedula, R. R. Microwave‐Assisted Synthesis and Biological Evaluation of Some New Pyrazolothiazoles via a Multicomponent Approach. ChemistrySelect 2019, 4 (33), 9878-9881.
CrossRef - Bariwal, J. B.; Trivedi, J. C.; Van der Eycken, E. V. Microwave irradiation and multicomponent reactions. Synthesis of Heterocycles via Multicomponent Reactions II 2010, 169-230.
CrossRef - Machado, I. V.; Dos Santos, J. R.; Januario, M. A.; Corrêa, A. G. Greener organic synthetic methods: Sonochemistry and heterogeneous catalysis promoted multicomponent reactions. Ultrasonics Sonochemistry 2021, 78, 105704.
CrossRef - Dastkhoon, M.; Ghaedi, M.; Asfaram, A.; Azqhandi, M. H. A.; Purkait, M. K. Simultaneous removal of dyes onto nanowires adsorbent use of ultrasound assisted adsorption to clean waste water: chemometrics for modeling and optimization, multicomponent adsorption and kinetic study. Chemical Engineering Research and Design 2017, 124, 222-237.
CrossRef - Rotstein, B. H.; Zaretsky, S.; Rai, V.; Yudin, A. K. Small heterocycles in multicomponent reactions. Chemical reviews 2014, 114 (16), 8323-8359.
CrossRef - Maddila, S.; Jonnalagadda, S. B.; Gangu, K. K.; Maddila, S. N. Recent advances in the synthesis of pyrazole derivatives using multicomponent reactions. Current Organic Synthesis 2017, 14 (5), 634-653.
CrossRef - Safari, J.; Nasab, N. H. Ultrasonic Activated Efficient Synthesis of Indenopyrazolones via a One-Pot Multicomponent Reaction. Polycyclic Aromatic Compounds 2021, 41 (7), 1383-1391.
CrossRef - Heravi, M. M.; Mirzaei, M.; Beheshtiha, S. Y. S.; Zadsirjan, V.; Mashayekh Ameli, F.; Bazargan, M. H5BW12O40 as a green and efficient homogeneous but recyclable catalyst in the synthesis of 4H‐Pyrans via multicomponent reaction. Applied Organometallic Chemistry 2018, 32 (9), e4479.
CrossRef - Cioc, R. C.; Ruijter, E.; Orru, R. V. Multicomponent reactions: advanced tools for sustainable organic synthesis. Green Chemistry 2014, 16 (6), 2958-2975.
CrossRef - Haji, M. Multicomponent reactions: A simple and efficient route to heterocyclic phosphonates. Beilstein journal of organic chemistry 2016, 12 (1), 1269-1301.
CrossRef - Zarganes‐Tzitzikas, T.; Chandgude, A. L.; Dömling, A. Multicomponent reactions, union of MCRs and beyond. The Chemical Record 2015, 15 (5), 981-996.
CrossRef

This work is licensed under a Creative Commons Attribution 4.0 International License.









