The Impact of Citric Acid Hydrolysis on Starch Functionality in Mangifera Indica of Sindoor Variety: A Comprehensive Analysis
Department of Food Science Technology and Nutrition, Periyar University, Periyar Palkalai Nagar, Salem - 636 011, Tamil Nadu, India.
Corresponding Author E-mail: parimala1996@gmail.com
DOI : http://dx.doi.org/10.13005/ojc/400130
Article Received on : 13 Sep 2023
Article Accepted on : 01 Jan 2024
Article Published : 23 Feb 2024
Reviewed by: Dr. Hema Saravanan
Second Review by: Dr. N. Shyamaladevi
Final Approval by: Dr. N. Uddin
The mango (Mangifera indica) is a significant tropical fruit crop that is cultivated primarily for its pulp. Mango stone kernels were classified as insufficiently utilized foods and were employed as a by-product in food production. The study aimed to modify the starch from mango kernels by acid hydrolysis with citric acid (CAH) and heat treatment (CAHT) at 120°C for 2.5 hours. The extracted starch was characterized using functional (DSC and RVA), chemical (yield, amylose content, amylopectin content, and pasting clarity) analysis and structural properties (SEM, XRD, and FTIR) were analyzed using standard procedures. The results on functional analysis reported that the gelatinization enthalpy and thermal stability of CAH starch were higher, and had lower peak temperatures with increased viscosity. CAH starch had a greater starch yield, pasting clarity was high, and low amylose and amylopectin were found in CAHT starch. Acid hydrolysis had little effect on the granule size or morphology (SEM), and infrared (FTIR) examination showed 16 chemical bands and functional groups. The results of the investigation showed that CAH of Mangifera indica starch had better structural, functional, and chemical properties than CAHT Mangifera indica starch. For the technological process, starch modification with citric acid is preferable, and cross-linking is preferable to esterification alone.
KEYWORDS:Chemical; Citric acid; FTIR; Starch; SEM; Thermal; viscosity
Download this article as:| Copy the following to cite this article: Kaliyappan R, Ramanathan P. The Impact of Citric Acid Hydrolysis on Starch Functionality in Mangifera Indica of Sindoor Variety: A Comprehensive Analysis. Orient J Chem 2024;40(1). |
| Copy the following to cite this URL: Kaliyappan R, Ramanathan P. The Impact of Citric Acid Hydrolysis on Starch Functionality in Mangifera Indica of Sindoor Variety: A Comprehensive Analysis. Orient J Chem 2024;40(1). Available from: https://bit.ly/48nCkf3n |
Introduction
Mango (M. indica) is a member of the Anacardiaceae family of flowering plants. Despite being widely grown throughout Southeast Asia, Mangifera indica was formerly believed to have originated in India1. Traditionally, M. indica plant parts have been used to treat a variety of medical diseases, such as gastrointestinal, genitourinary, ocular, and respiratory disorders. These parts contain diverse types. Mangoes with a distinct appearance with shades of green and red, the sindoor variety is also known as Rajgira in Karnataka. The fruit is soft and juicy. Because of its remarkable sweetness and flavor, it is also known as “Honey Mango.” Because of its vermillion hue, which is similar to Sindoor, the term Sindhura originated2.
The discarded kernel parts of Mangifera indica are an important source of phytochemicals, carbohydrates, lipids, proteins, vitamins, and protective and nutritional fibers against diseases linked to oxidative stress that impact human health3. Even though these seeds may contain nutrients and bioactive chemicals, many consumers and fruit growers throw them away as waste when making juices, jams, and snacks. During industrial processing, this produces a huge amount of garbage, which causes serious disposal problems. Thus, utilizing trash to produce commodities with added value is crucial to managing waste from food processing. The mango seed kernel’s approximate composition is as follows: 2.58% ash, 11.55% fat, 6.02% protein, 10.60% crude fiber, 59.34% carbs, and 9.89% moisture4.
Most plants primarily obtain their carbohydrates from starch, which is found in large quantities. Finding new sources of this kind of polysaccharide from different plants or fruits is becoming more and more important due to the expanding need for starch in a variety of industries, including food, pharmaceuticals, paper, glue, and textiles. The utilization of starch derived from many plant sources, including rice, wheat, corn, and potatoes, has been the subject of numerous attempts5.
Citric acid modification of starch can decrease swelling power while boosting solubility; this is assumed to be because starch hydrolyses in acid and produces short chains, which increases the hydration capacity of the starch chain hydrolysis process6. The hydrolysis of the amylose and amylopectin chain acids that result from heating at a higher temperature (>80°C) in the presence of citric acid influences the retrogradation and gel strength of the maize starch gel. Generally regarded as harmless, citric acid is a naturally occurring poly-carboxylic acid that may be isolated from a variety of fruits. Significant changes in starch’s properties, such as granule morphology, rheological and thermal properties, susceptibility to retrogradation and syneresis, solubility and swelling power, and susceptibility to amylolytic enzyme action, are brought about by the esterification and crosslinking of starch with citric acid. Citric acid and heat treatment are combined to produce durable, water-resistant coatings with superior mechanical qualities7.
The fact is commonly recognized that different starch varieties have varied temperature profiles for gelatinization, which affects the gelling properties of high and low-molecular starch granules. Compared to bigger granules, the fraction of small-sized starch granules has a higher phosphorus content. The average granule sizes of starches extracted from various types of starchy foods are closely connected with physical and chemical features such as light transmission, amylose content, swelling power, and water-sorption ability8. However, there hasn’t been much research done on the process of extracting starch from Mangifera indica by-products such as seeds. The investigator wants to investigate the chosen sindoor variety-based starch extraction research as they haven’t been done before. Thus, the M. indica seed kernel was used in this investigation for the extraction and characterization of starch. Based on the aforementioned viewpoint, the study aimed to determine the effects of citric acid hydrolysis and heat treatment on the functional, chemical, and structural characterization of Mangifera indica starch.
Materials and Methods
Materials
Mangoes (Mangifera indica L.) of the Sindoor variation were purchased in a local marketplace in Krishnagiri, Salem District, Tamil Nadu, India. The starchy substance used in the experiment of this study has been derived from the kernels found in the central portion of the mango fruit. The extraction and analyses were accomplished in the laboratory of the Bio Vision Research Centre, Thanjavur, Tamil Nadu.
Preparation of modified starch
Citric acid treatment (CAH)
Employing the technique described by Wickramasinghe et al., (2009)9, mango kernel starch was extracted. Five percent (0.26 mol/L) citric acid was used for dissolving alkali-treated mango kernel starch before it was processed for sixty minutes at 45°C in a boiling water bath with steady stirring. After hydrolysis, the resulting solution was quickly annihilated to bring the pH to 7.0 by adding 0.1 M sodium hydroxide after 1 M sodium hydroxide. Before filtering, starch had been air dried for 48 hours in a convection chamber after being washed numerous times in deionized water10.
Citric acid with heat treatment (CAHT)
All of the samples underwent citric acid impregnation and 103°C oven drying. Two subgroups of each group were created, one of which received pretreatment at 120°C for 2.5 hours. Before analysis, all of the materials were ground to a size lower than 100 mesh
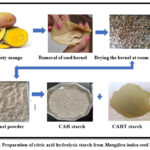 |
Figure 1: Preparation of citric acid hydrolysis starch from Mangifera indica seed kernel. |
Functional properties of starch
Thermal Properties (DSC)
The thermodynamic properties of starches were investigated using a differential scanning calorimeter equipped with a thermal analysis recording unit (DSC-821e, Mettler Toledo, Schwarzenbach, Swiss). A starch-based sample (3.5 mg, dry weight) was put into a 40-l capacity aluminum pan (ME- 27331, Mettler), and water that had been distilled was then added utilizing a Hamilton micro-syringe (Hamilton Micro syringe, St. Louis, MO, USA). The result was a starch suspension containing water consisting of seventy percent water. Materials were sealed with silicone and allowed to stand for two hours at normal temperature before DSC measurements. To set up the differential scanning calorimeter (DSC) analyzer using indium, it was heated from 20 to 100°C at an average speed of 10°C/min. The beginning temperature (To), the peak temperature (Tp), the completion temperature (Tc), and the enthalpy of gelatinization (ΔHgel) were all calculated automatically. The elevation of the peak index (PHI) was calculated using the ratio of ΔHgel to (Tp – To), and the amount of gelatinization range (R) was (Tc – To)11.
Rapid Visco Amylograph analysis (RVA)
The starch levels have been generated by mixing 28 g of distilled water with 8% (or around 2.24 g) of starch in an aluminum sample container. Then, materials were evaluated using a Rapid Visco Analyzer (made by Newport Scientific in Australia). The specimens were maintained at 50°C for 1 minute, then preheated to 95°C at 6°C per minute for 2.5 minutes, and finally dropped to 50 degrees Celsius at 6°C per minute for an additional 2 minutes12.
Chemical properties of starch
Considering the described process, the chemical characteristics of mango kernel starch, including recovery yield, amylose content, amylopectin content, and pasting clarity, were examined.
Recovery Yield
The powdered air-dry starch was then used to compute the recovered yield using the subsequent method13.
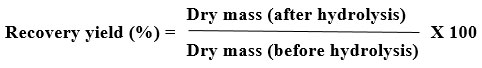
Amylose content
A colorimetric iodine affinity procedure was used to determine the amylose concentration. 10 minutes were devoted to cooking 0.1 g of the starch specimens in a hot water bath, along with 1 ml of ethanol, 9 ml of 1 N sodium hydroxide, and chilling. To measure the absorbency (A) at 620 nm, a minor quantity of the mixture (5 ml) was mixed with 1 ml of 1 N acetic acid solution and 2 ml of the solution of iodine14. The amount of amylose has been determined as follows:
Amylose content (%) = 3.06 X A X 20
Where, A= Absorbance value
Amylopectin content
Amylopectin in tested starch was computed by difference using the following formula16:
Amylopectin (%) = 100% – amylose (%)
Pasting clarity
Paste clarity was defined as the optical transmission of a 1% starch paste evaluated at 650 nm. For 30 minutes, boiling (about 98°C) starch dispersion was heated to encourage thorough gelatinization of the granules. Following the cooling of the pastes to 20°C, the transmittance was calculated. The outcomes were three repetitions’ averaged15.
Structural properties of starch
Scanning Electron Microscopy (SEM)
The SEM photographs of the films were taken with a QUANTA FEG-FEI 450 microscope using scanning electronic microscopy (SEM). The specimens were placed on the aluminum stub employing carbon-coated double-sided sticky tape and subsequently plated with a 20 nm thick protective layer of gold using a Quorum QT150ES metallizer. The specimens were then examined using a 5 kV voltage for acceleration and a 2000x magnifying.
X-ray diffraction (XRD)
To construct the X-ray diffraction pattern, starch was employed as a powder with around 16% moisture. The Shimadzu Corporation X-ray Diffractometer, model XRD-6000 with graphene monochromator, operated beneath the following conditions: the radiation from CuK (= 1.5405), at an output voltage of 40 kV, 30 mA, with a scanning velocity of 1.2° min-1, at a degree of 2, ranging from 3 – 35° and spacing of 0.02°. The degree of crystallinity was estimated quantitatively16-18, by the equation below:
Dc (%) = Ac (Ac + Aa) × 100
Where Dc is the degree of crystallinity, Ac is the crystalline area on the X-ray diffractogram, and Aa is the amorphous area on the X-ray diffractogram.
Fourier Transform Infrared Spectroscopy (FTIR)
The Fourier transform infrared (FTIR) measurement was conducted using a 64-scan, 4 cm-1 resolution Shimadzu FT-IR 8400 spectroscopy (Shimadzu, Japan). To obtain the spectrum, 100 mg of dry KBr and 2 mg of the specimen (on a dehydrated base) were combined uniformly to form KBr pellets. The spectra’s wave number amplitude fell around 400 and 4000 cm-1 19.
Statistical analysis
The software application SPSS 25th version was employed to calculate the samples’ mean standard deviation for mango kernel starch. For each, three separate analyses were carried out.
Results and Discussion
Effects of citric acid modification on thermal properties of mango kernel starch
Table 1: Effects of citric acid modification on thermal properties of Mangifera indica starch
|
DSC |
To (ºC) |
TP (ºC) |
TC (ºC) |
∆Hgel (J/g-1) |
|
CAH starch |
61.3±0.65 |
63.6±0.46 |
67.6±0.74 |
4.3±0.1 |
|
CAHT starch |
52.65±0.85 |
58.89±0.27 |
71.34±0.92 |
2.20±0.3 |
CAH -Citric acid hydrolysis; CAHT – Citric acid hydrolysis with heat treatment; To – onset temperature, TP – Gelatinization temperature; Tc – conclusion temperature, ∆Hgel – enthalpy temperature
The onset temperature (To), peak temperature (Tp), conclusion temperature (Tc), and endothermic enthalpy (∆Hgel) of the DSC thermograms are all described in Table 1 and Figure 1. CAH starch exhibited a high onset temperature (61.3°C), peak temperature (63.6°C), and also exhibited high enthalpy (4.3j/g-1) and CAHT starch exhibited a high conclusion temperature (71.31°C). The first peak is called the cooperative melting peak, and the second is the “true” melting process. The presence of both is attributed to the water content absorbed by the starch granules. The onset temperature for gelatinization attributed to the first transition is considered low when compared to other starches. It was found higher temperatures for the gelatinization of 5 mango cultivars from India with similar water content. According to Jane and co-workers starches with lower onset gelatinization temperature presented the shortest average amylopectin branch chain length and the largest proportion of short chains19. Mango kernel starch’s increased capacity for hydration may have reduced the amount of water available for starch gelatinization, which explains why citric acid added to the starch pastes and made with heating of mango kernel starch had lower ΔHgel readings.
Heat and citric acid significantly reduced the temperature at which the gelatinization is formed. At the beginning of the gelatinization, the temperature decreased from 61.3 °C to 52.65 °C, and at the end of the temperature rapidly increased from 67.6 °C to 71.34 °C. The difference in mango kernel starch gelatinization concerning citric acid dose and heat treatments across the above citric acid range is significant. The significant increase in gelatinization temperatures and decrease in enthalpy may be due to the action of hydrogen oxygenation (H3O+) on the starch chains and the double helices in amorphous granules20.
When citric acid is added to heat-treated starch, enthalpy decreases significantly. The enthalpy decreases with a decrease in onset temperature a decrease in gelatinization temperature and an increase in conclusion temperature (Tc). For mango kernel starch, the enthalpy values ranged between 4.3 and 2.20 j/g. The energy required for the progression of gelatine is an estimate of the degree of compassion, the quantity, and the destruction of the structural organization inside granules. Low enthalpy indicates reduced stability and structural disorder of the crystal. When large groups of hydroxypropyls are added to amorphous components of starch, it breaks down a double helix, which increases structural flexibility and lowers its melting point. Acid treatment also breaks down intracellular and intermolecular hydrogen bonds in the granules, reducing crystallinity, increasing water accessibility to the starch granules, and lowering enthalpy21, 22.
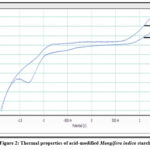 |
Figure 2: Thermal properties of acid-modified Mangifera indica starch. |
The thermal properties of M. indica kernel starch is presented in Figure 2. A decrease in gelatinization temperature and enthalpy could be observed in CAHT starch which may be due to the modified starches that underwent acid hydrolysis. Starch undergoes physicochemical changes during acid hydrolysis, maintaining its granular structure. The crystalline parts of starch particles are more resistant to acid degradation than their amorphous counterparts. Gelatinization temperature and enthalpy are shown after acid treatment. As the straight chain concentration increases, the probability that the acid-liquefied starch will reduce and increase the amorphous region. A higher degree of hydrolysis increased the crystallinity fraction, which increased the gelatinization temperature. This may explain why acid-treated starches have high Tp and Tc values. Acid-modified potato starch and sweet potato starch gave comparable results23.
Effects of citric acid modification on pasting properties (RVA) of Mangifera indica starch
Table 2: Effects of citric acid modification on pasting properties (RVA) of Mangifera indica starch
|
Pasting parameters |
Peak viscosity (cP) |
Tough viscosity (cP) |
Breakdown viscosity (cP) |
Final viscosity (cP) |
Setback viscosity (cP) |
Peak time (min) |
Pasting temperature (oC) |
|
CAH starch |
2396.50 |
1274.01 |
1269.50 |
2481.50 |
1074.50 |
4.76 |
77.27 |
|
CAHT starch |
2382.50 |
1354.01 |
1358.01 |
2385.50 |
1172.50 |
4.18 |
4.18 |
CAH -Citric acid hydrolysis; CAHT – Citric acid hydrolysis with heat treatment
Table 2 displays the pasting characteristics of Mangifera indica starch. The peak viscosity of CAH starch was found to be higher at 2393.50 cP than that of CAHT starch at 2382.50 cP, indicating a higher potential for starch swelling in the former. There were higher breakdown viscosities (1358.01 cP) and tough viscosities (1354.01 cP) with CAHT starch. Compared to CAHT starch, CAH starch has a much greater ultimate viscosity (2481.50cP). According to Zaidul et al., (2007) stated that the setback viscosity of CAHT starch was higher (1172.50 cP) than that of CAH starch, suggesting a greater propensity for amylose to retrograde24. Compared to CAH starch, CAHT starch exhibited a lower pasting temperature (4.18 minutes). Significant crystalline starch breakdown is likely to occur since citric acid has a lower strength than hydrochloric acid. Citric acid creates an anhydride and dehydrates when heated. With more heating, citric acid can dehydrate and form cross-links with other starch molecules25.
Ali et al. (2020) found in their study that Heat-modified lotus seed starch used a similar temperature increase. The peak viscosities of CAHT starch decreased after heat treatment due to amylose-lipid complexes or interactions with amylopectin chains, which prevented the spreading and swelling of starch granules. Citrate was used instead of starch to avoid gelatinization and swelling during the RVA assay26. In the RVA experiment, heat treatment significantly reduced starch swelling and adhesion to citric acid due to higher citrate substitution and concomitant heat activity. As a result of the citric acid modification, Hausa potato starch has increased thermal stability and viscosity, which favors its use as an edible component in preserves, as a binder in meat and bread, and as a texture in sweets and dairy products27.
Effects of citric acid modification on Chemical properties of modified Mangifera indica starch
Table 3: Effects of citric acid modification on Chemical properties of modified Mangifera indica starch
|
Modifications of starch |
Yield (%) |
Pasting Clarity (%) |
Amylose content (%) |
Amylopectin content (%) |
|
CAH starch |
15.1±1.0 |
11.1±0.2 |
27.5±0.5 |
80.5±0.8 |
|
CAHT starch |
16.3±0.2 |
9.8±0.4 |
25.10±0.3 |
74.90±0.7 |
CAH -Citric acid hydrolysis; CAHT – Citric acid hydrolysis with heat treatment
According to the chemical properties of starches, CAHT starch had a high starch yield (16.3%), whereas CAH starch had a high pasting clarity (11.1%). Our Mangifera indica starch production was much lower than that of Chowdary et al. (2000)28 and Silva et al. (2013), who found yield percentages of 55% and 59.82%, respectively29. High levels of amylose (27.5%) and amylopectin (80.5%) were found in the CAH starch. According to Robyt’s (2008) research, starch typically comprises 20–30% amylose and 70–80% amylopectin30. Our analysis simply showed the same ratio of amylose and amylopectin content.
The clarity of starch paste is affected by the number of non-starch molecules, particularly proteins and lipids, as well as the amylose-to-amylopectin ratio. Furthermore, the method used to generate the starch paste, specifically the temperature, time, and rate at which the starch solution is heated, dictates how clear the starch paste will seem after storage since it affects how the starch chains interact31.
Singh et al., (2003) discovered substantial differences in the amounts of citric acid and heat treatment of starch32. Because of the lyses of amylopectin fractions, the CAH starch amylose content (27.5%) was high. Amylose content and granule form both influences how easily gelatinization is caused by heating. Singh observes that because the hydrogen bonds between the chains are so weakly formed, increasing the amylose content facilitates this process. Water absorption, gelatinization and pasting, retrogradation, and susceptibility to enzymatic assault are all affected by amylose/amylopectin concentration and granule size33.
These chemical characteristics of starch made it clear that CAH starch can be used in the food industry due to its high amylose content. Starch is normally used in industries other than thickening, like drug delivery34, dietary fiber35, and emulsion stability36.
Effects of citric acid modification on SEM analysis of Mangifera indica starch
CAH starch granules have regular, elliptical, and polygonal forms, and their diameters are around 2 m (Figure 3A). The starch granules underwent severe surface damage and severe rupture after being heated and subjected to citric acid treatment, as shown in Figure 3B, resulting in a large number of fragments. Due to granule disintegration, CAHT starch shrinks. Even though the starch particles were now noticeably smaller, there were now more of them altogether. It is widely recognized that during the acid hydrolysis with heat treatment process, the amorphous areas of the starch particles were preferentially eliminated. The amorphous zone was thus discovered inside the mango kernel starch particle, according to the SEM data. Starch granules break apart and become smaller as a result.
In 3A, the structure of starch was good in ellipsoidal shape and was shown clearly, and in 3B starch granules were seen as a mass structure and its s shape was not seen. As per this literature, the addition of citric acid did not alter the polyhedral structure of rice starch particles, as the concentration increased, the surfaces of some granules showed faint erosion marks, which was analogous to our CAH starch SEM analysis37. In comparison to other previous studies, it was also discovered that citric acid modification did not affect the granule form of other types of starch, including maize, sweet potato, yam, potato, cassava, banana, and bean. Citric acid hydrolysis caused some starch granules to become rougher and form small pores or fissures and only in certain percentages38.
 |
Figure 3: SEM analysis of citric acid-modified Mangifera indica starch; 3A. SEM analysis of CAH starch; 3B. SEM analysis of CAHT starch. |
According to the findings of CAHT starch, when starch granules are heated in a liquid, they inflate and rupture, which causes the liquid to thicken. This is the process of gelatinization. The liquid materials are absorbed by the starch granules during heating at 60°C. The process of gelatinization reached its peak at boiling point (100°C), at which point the molecules burst, releasing amylose and amylopectin and enabling even more liquid to be absorbed. This means that starch treated with CAHT gelatinizes very quickly, while starch treated with amylase breaks down slowly or not at all because of denaturation.
Significant variability was seen in high-amylose starch, which was composed of three distinct granule types: elongated, aggregated, and individual granules. Compared to regular maize, the individual granules were rounder in shape, smaller, and had smoother surfaces. Granules that were extended and aggregated had lumpy, uneven forms that distinguished them from individual granules. Each of these granules has a surface devoid of pores39. This investigation contradicted our findings because the surface of the individual CAH starch granules was smoother.
Effects of citric acid modification on XRD spectrum values of Mangifera indica starch
Table 4: Effects of citric acid modification on XRD spectrum values of Mangifera indica starch
|
Starch modifications |
Pos (°2TH) |
Height (cts) |
FWHM left (2Th) |
d-spacing (A) |
Diffraction Intensity (%) |
Degree pattern |
|
CAH starch |
30.159 |
75.68 |
0.275 |
2.963 |
100 |
A type |
|
32.32 |
43.86 |
0.206 |
2.769 |
57.95 |
||
|
33.42 |
37.72 |
0.482 |
2.680 |
49.85 |
||
|
35.35 |
62.62 |
0.275 |
2.539 |
82.75 |
||
|
37.90 |
63.20 |
0.206 |
2.373 |
83.51 |
||
|
41.481 |
24.59 |
0.551 |
2.17 |
32.49 |
||
|
48.34 |
23.88 |
0.413 |
1.88 |
31.55 |
||
|
CAHT starch |
30.159 |
74.68 |
0.255 |
2.953 |
100 |
A type |
|
32.32 |
43.86 |
0.205 |
2.767 |
58.95 |
||
|
33.427 |
37.62 |
0.482 |
2.680 |
49.85 |
||
|
37.90 |
63.10 |
0.216 |
2.353 |
83.51 |
CAH -Citric acid hydrolysis; CAHT – Citric acid hydrolysis with heat treatment; FWHM -Full width at half maximum
The starch granules are semi-crystalline white particles, insoluble in water at room temperature. The size and shape of the granules are specific to each plant species. In a very simplified way, the organization of the starch grain results from the arrangement of amylose and amylopectin in amorphous and crystalline zones arranged concentrically from the hilum. The crystallinity of the starches is mainly due to the double helix chains of amylopectin and the cohesion of the crystalline zones is ensured by intermolecular hydrogen bonds. The X-ray diffraction patterns of CAH and CAHT starch are presented in Figure 4. The corresponding X-ray diffraction parameters and crystallinity level calculated from the ratio of the diffraction peak area and the total diffraction area are given in Table 4. The scattering angle, at which the diffraction intensities can be observed, was 2θ, and the d spacing was used to discriminate the planes of different sites. The XRD pattern of CAH starch contains diffraction peaks (2θ) at 30.159°, 32.32°, 33.42°, 35.35°, 37.90°, 41.481° and 48.34°, which correspond to the A-type crystalline structure shown in figure 3a. The XRD pattern of CAHT starch contains diffraction peaks (2θ) at 30.159°, 32.32°, 33.427, and 37.90°, which correspond to the A-type crystalline structure shown in Figure 3b.
The relative crystallinity of the starches demonstrated a crystallinity ranging from 31.55 to 100% (Table 4), which agreed with previous data on potatoes40. No significant change in the type of crystalline pattern was observed as a result of CAH starch compared to the CAHT starch, as the major peaks were similar. However, the relative intensity of the predominant diffraction peaks of starch granules was not detected after citric acid hydrolysis and heat treatment at 120°C corresponding to nil crystallinity. There was a decrease in the relative crystallinity percentage of starch granules were heated in moist conditions. This may be due to the destruction of the crystalline portion showed a mass structure granule as evidenced in the SEM images (to be discussed above). According to Gavrilova et.al., (2019) the crystallinity degree significantly affects the physicochemical properties such as hardness, density, transparency, and reactivity. However, these properties do not depend solely on crystallinity degree and are also affected by granule size and the type of crystal structure41.
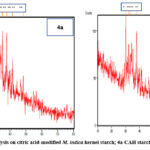 |
Figure 4: XRD analysis on citric acid-modified M. indica kernel starch; 4a-CAH starch 4b- CAHT starch. |
Citric acid-modified Mangifera indica starch exhibits a distinctive A-type diffraction pattern, as apparent in Figures 4a and 4b. Diffractions increased in all acid modification processes. Citric acid42 is esterified even in the partly crystalline and amorphous starch granule phases. The heat treatment and citric acid raised the starch’s relative crystallinity and increased its susceptibility to citric acid in the case of mango kernel starch. Amylose forms amorphous regions in starch, but amylopectin forms a double-helix structure to control crystallization.
Effects of citric acid modification on FTIR analysis of Mangifera indica starch
Fourier transform infrared spectroscopy (FTIR) method was used to characterize the different functional groups such as lipids, proteins, nucleic acids, and polysaccharides present on the cell membrane and determine their functions43. The Fourier transform infrared bands of Mangifera indica starch are shown in Figure 5. The peak at 900–950 cm−1 in the infrared spectrum of all the starch samples is evidence of the vibration of the glycosidic linkage. A distinctive peak was aroused at 1648 cm-1, which may be due to the presence of the water molecules bound to the starch44. A strong, broad band between 3000 and 3700 cm−1 was noticed which corresponds to the O-H stretch. The sharp band at 2932 cm−1 is characteristic of C-H stretches associated with the ring hydrogen atoms. The percentage intensity of the C-H stretch of acid-modified starches was decreased slightly compared to the CAHT starch. Intensity changes in the C-H stretch range may be attributed to the change in the amylose and amylopectin content of the starch molecule. Dutta et al., (2011) indicated gradual changes in the positions of the peak between 2970 and 2910 for acid-treated jackfruit seed starch. The peaks at 1377 cm-1 and 1333 cm-1 might be connected to the displacement structure of O-C-H, C-C-H, and C-O-H. This matched up with the consequences of our mango kernel starch hydrolysis in citric acid45.
An additional peak in a modified condition was observed at about 1735 cm-1 and was associated with the distinct ester group. This demonstrates how the esterification reaction between the functional groups of citric acid (-COOH) and starch (-OH) shapes ester groups. Confirmed findings point to a chemical shift in the cross-linking of the soy residue brought on by the absorption of citric acid. Pornsuksomboon et al., (2016) reported similar outcomes regarding the film’s incorporation of citric acid-producing cross-linking46.
C-H bending was seen in both CAH and CAHT starches (850–1000 cm–1). A peak in the 1742 cm-1 band was linked to the synthesis of carboxyl and carbonyl ester, indicating that starch and citric acid interact chemically. This implies that a wide range of vibrational modes are involved in the interactions between citric acid and starch 47, 48.
 |
Figure 5: FTIR analysis of citric acid-modified Mangifera indica starch; 5a. CAH starch; 5b. CAHT starch. |
Conclusion
The structural, chemical, and functional properties of Mangifera indica starch were then observed subsequently being heated and modified through citric acid. It was discovered that these starches differ greatly from one another. having greater thermal gelatinization values of To (61.3°C), Tp (63.6°C), Tc (67.6°C), and ΔHgel (4.3°C), respectively, the CAH starch that showed delayed retrogradation. In CAHT starch, peak viscosity, and pasting temperature were more important. CAH starch had a significant starch yield, paste clarity, and concentration of amylose and amylopectin compared to other methods. The chemical properties of modified starches were considerably different from those of unmodified starches. CAH starch granules retained their ellipsoidal shape. CAH starch led to a high level of crystallinity, whereas CAHT starch resulted in X-ray diffraction of A-type starch. Through FTIR analysis both the starches exhibited that citric acid modification gives out the functional compounds present in it. The outcomes of the study demonstrated that starch granules exposed to citric acid had better functional, chemical, and structural qualities than those treated with heat. Since modified starches are used in almost every starch application, including as a binding agent, a thickening agent, a stabilizer, or for emulsion formation in food products, as well as an adhesive for pharmaceuticals in medical products, the food processing industry has the potential to use acid-modified Mangifera indica starch.
Acknowledgement
The authors express their gratitude to Periyar University in Salem, Tamil Nadu, for providing the tools required to carry out this study.
Authors contribution statement
RK came up with the concept and did the data collection for this study. This article’s authors, RK and PR, were able to create it after carefully analyzing the data inputs. Before submitting their work for publication, all authors discussed the technique and results interpretation.
Conflict of Interest
The principal author and co-author of this paper were not upfront about any conflicts of interest.
Funding Sources
There is no funding sources.
References
- Khalid F.; Nawaz H.; Hanif M.A.; Rehman R.; Al-Sadi A.M. Amsterdam, Oxford, Cambridge MA: Elsevier., 2020, 495–508.
CrossRef - Shah K.A.; Patel M.B.; Patel R.J.; Parmar P.K. Pharmacogn Rev., 2010, 4(7), 42–48. doi:10.4103/0973- 7847.65325.
CrossRef - Lasano N.F.; Ramli N.S.; Hamid A.H.; Karim R.; Pak Dek M.S.; Shukri R.; Oriental Pharmacy and Experimental Medicine., 2019, 19 (3), 277–286. https://doi.org/10.1007/s13596-019-00383-z.
CrossRef - Omotubga S. K.; Kehinde A. S.; and Olayinka O.O. Intern. J. Res. Chem. Environ. 2012, 2:244–245.
- Alcazar-Alay S.C.; and Meireles M.A.A. Food Science and Technology., 2015, 35, 215-236. https://doi.org/10.1590/1678-457X.6749
CrossRef - Singh H.; Chang Y.H.; Lin J.H.; Singh N.; and Singh N. Food Res. International., 2011, 44, 2949-2954.
CrossRef - Da-Costa-Rocha I.; Bonnlaender B.; Sievers H.; Pischel I.; and Heinrich M. Food chemistry., 2014, 165, 424-443.
CrossRef - Singh J.; and Singh N. Food Chemistry., 2001, 75 (1), 67–77.
CrossRef - Wickramasinghe, N. S.; Manavalan, T. T.; Dougherty, S. M.; Riggs, K. A.; Li, Y.; Klinge, C. M. Nucleic Acids Res., 2009, 37(8), 2584-2595.
CrossRef - Zambrano, F.; Camargo, C.R.O. Braz J Food Technol. 2002, 4,147–154.
- Kruger, T. F.; Acosta, A. A.; Simmons, K. F.; Swanson, R. J.; Matta, J. F.; Veeck, L. L.; Brugo, S. Urology., 1987, 30(3), 248-251.
CrossRef - Dutta, H.; Paul, S.K.; Kalita, D.; Mahanta, C.L. Food Chemistry., 2011, 129(2), 284-291.
CrossRef - , H. Y.; Park, D. J.; Kim, J. Y.; Lim, S. T. Carbohydr. Polym., 2013, 98(1), 295-301.
CrossRef - Juan, G.; Luis, A.; David, B. Starch., 2006, 58, 300-307.
CrossRef - Lawal, O. S.; Adebowale, K.O. Carbohydr. Polym., 2005, 60(3), 331-341.
CrossRef - Andrade, M. M. P.; de Oliveira, C. S.; Colman, T. A. D.; da Costa, F. J. O. G.; Schnitzler, E. J. Therm. Anal. Calorim., 2014, 115(3), 2115-2122.
CrossRef - Beninca, C.; Colman, T. A. D.; Lacerda, L. G.; Carvalho Filho, M. A. S.; Bannach, G.; Schnitzler, E. Thermochimica Acta., 2013, 552, 65-69.
CrossRef - Colman, T. A. D.; Demiate, I. M.; Schnitzler, E. J. Therm. Anal. Calorim., 2014, 115(3), 2245-2252.
CrossRef - Chi, N. C.; Shaw, R. M.; De Val, S.; Kang, G.; Jan, L. Y.; Black, B. L.; Stainier, D. Y. Genes Dev., 2008, 22(6), 734-739.
CrossRef - Hoover, R.; Manuel, H. A. Food Chem., 1995, 53(3), 275-284.
CrossRef - Liu, R. Y.; Parelius, J. M.; Singh, K. Ann Stat, 1999, 27(3), 783-858.
CrossRef - Woggum, T.; Sirivongpaisal, P.; Wittaya, T. Food Hydrocoll., 2015, 50, 54-64.
CrossRef - Singh, S.; Raina, C. S.; Bawa, A. S.; Saxena, D. C. J Food Sci., 2005, 70, E373–E378. doi: 10.1111/j.1365-2621. 2005. tb11441. x.
CrossRef - Zaidul, I. S. M., Norulaini, N. N., Omar, A. M., Yamauchi, H., and Noda, T. Carbohydrate polymers., 2007, 69(4), 784-791.
CrossRef - Wing, R.E. Starch/Starke. 1996, 48, 275–279. doi: 10.1002/star.19960480709.
CrossRef - Ali, N. A.; Dash, K. K.; Routray, W. Food Chem., 2020, 319, 126513.
CrossRef - Navaf, M.; Sunooj, K.V.; Aaliya, B.; Sudheesh, C.; Akhila, P.P.; Sabu, S.; Sasidharan, A.; George, J. Int. J. Biol. Macromol., 2021, 182, 554–563.
CrossRef - Chowdary, G. V.; Ramesh, M. N.; Prapulla, S. G. Process Biochem., 2000, 36(4), 331-339.
CrossRef - Silva, E.; Birkenhake, M.; Scholten, E.; Sagis, L. M. C.; Van der Linden, E. Food Hydrocoll., 2013, 30(1), 42-52.
CrossRef - Robyt JF. In: Fraser-Reid BO, Tatsuta K, Thiem J, editors. Glycoscience. Berlin, Heidelberg: Springer., 2008, 1437–72.
CrossRef - Singh, J; Singh, N. Food chemistry., 2001, 75(1), 67-77.
CrossRef - Singh, N.; Singh, J.; Kaur, L.; Sodhi, N.S.; Gill, B.S. Food Chem., 2003, 81, 219–231. doi: 10.1016/S0308-8146(02)00416-8.
CrossRef - Bajer, D., Kaczmarek, H., & Bajer, K. Carbohydrate polymers., 2013, 98(1), 477-482.
CrossRef - Faisal M.; Kou T.; Zhong Y.; and Blennow A. Polymers., 2022, 14(6), 1235.
CrossRef - Li B.; Yang W.; Nie Y.; Kang F.; Goff H. D.; and Cui S. W. Food Hydrocolloids., 2019, 94, 48-56.
CrossRef - Guo, B., Hu, X., Wu, J., Chen, R., Dai, T., Liu, Y., and Liu, C. Food Hydrocolloids., 2021,111, 106377.
CrossRef - Huo, Y.; Zhang, B.; Niu, M.; Jia, C.; Zhao, S.; Huang, Q.; Du, H. Int. J. Biol. Macromol., 2018, 116, 793–800.
CrossRef - Falade, K.O.; Ayetigbo, O.E. Food Hydrocoll., 2015, 43, 529–539.
CrossRef - Cai C.; Wei C. Carbohydr Polym., 2013, 92:469–478. doi: 10.1016/j.carbpol.2012.09.073.
CrossRef - Vamadevan V.; Blennow A.; Buleon A.; Goldstein A.; Bertoft E. Starch/Staerke., 2018, 70, 1700240.
CrossRef - Gavrilova K.V.; Bychkov A.L.; Bychkova E.S.; Akimenko Z.A.; Chernonosov A.A.; Kalamber Y.A.; Lomovskii O.I. Foods Raw Mater., 2019, 7, 255–263.
CrossRef - Shaikh, F.; Ali, T.M.; Mustafa, G.; Hasnain, A. Int. J. Biol. Macromol., 2019, 135, 314–327.
CrossRef - Dogan A.; Siyakus G.; and Severcan F. Food Chemistry, 2007. 100 (3), 1106-1114.
CrossRef - Fang J.M.; Fowler P.A.; Tomkinson J.; Hill C.A.S. Carbohydr Polym., 2002, 47:245–252.
- Dutta H.; Paul S.K.; Kalita D.; Mahanta C.L. Food Chem., 2011, 128:284–291.
CrossRef - Pornsuksomboon, K.; Hollo´, B.B.; Sze´cse´nyi, K.M.; Kaewtatip, K. Carbohydr. Polym., 2016, 136,107–112.
CrossRef - López-Córdoba, A.; Estevez-Areco, S.; Goyanes, S. Carbohydr. Polym., 2019, 215, 377–387.
CrossRef - Mansur, H.S.; Sadahira C.M.; Souza, A.N.; Mansur, A.A.P. Mater. Sci. Eng. C., 2008, 28, 539–548.
CrossRef

This work is licensed under a Creative Commons Attribution 4.0 International License.










