Synthesisis And Applications of Natural Polysaccharide Based Ion Exchanger, Guar Gum 2-Amino-3(4-Imidazolyl) Propanoic Acid (Gaipa) Resin for Removal of Toxic Metal Ions From Industial Efflunt’s of Steel Industry, Jodhpur, Rajasthan.
Department of Chemistry, Jai Narain Vyas University, Jodhpur, Rajasthan India.
Corresponding Author E-mail: areshvikram04@rediffmail.com
DOI : http://dx.doi.org/10.13005/ojc/400136
Article Received on : 25 Dec 2023
Article Accepted on :
Article Published : 16 Feb 2024
Reviewed by: Dr. Tapashi Ghosh Roy
Second Review by: Dr. N Raman
Final Approval by: Dr. Dinesh Chand Sharma
Natural Guar gum based resin which containing 2-amino, 3-(4-imidazolyl) propanoic acid as a functional group has been synthesized. This ion exchange GAIPA resin was used for removal of toxic metal ions from industrial effluents using the principle of ion exchange mechanism. GAIPA resin was used for removal of metal ions from reference solution containing metal ions and industrial effluents of steel industry, Jodhpur, which contained metal ions. Metal ions show maximum adsorption on GAIPA resin at pH 4-6.On this pH, resin have highest Kd value.
The sequence of maximum adsorption rate of metal ions from references solutions on GAIPA resin were follow as Pb(II)>Cd(II)>Fe(II)>Cu(II)>Zn(II)>Ni(II)>Co(II)>Cr(III) and from industrial effluents follow as Fe(II)>Pb(II)>Zn(II)>Cu(II)>Cd(II). The guar gum based resin GAIPA was characterized on the basis of ion exchange capacity and FTIR.
Adsorption; FTIR; Heavy Metal Ions; Ion Exchange Resin; Industrial Effluents; Guar Gum
Download this article as:| Copy the following to cite this article: Singh I, Singh P, Singh A. V. Synthesisis And Applications of Natural Polysaccharide Based Ion Exchanger, Guar Gum 2-Amino-3(4-Imidazolyl) Propanoic Acid (Gaipa) Resin for Removal of Toxic Metal Ions From Industial Efflunt’s of Steel Industry, Jodhpur, Rajasthan. Orient J Chem 2024;40(1). |
| Copy the following to cite this URL: Singh I, Singh P, Singh A. V. Synthesisis And Applications of Natural Polysaccharide Based Ion Exchanger, Guar Gum 2-Amino-3(4-Imidazolyl) Propanoic Acid (Gaipa) Resin for Removal of Toxic Metal Ions From Industial Efflunt’s of Steel Industry, Jodhpur, Rajasthan. Orient J Chem 2024;40(1). Available from: https://bit.ly/3I3l1FG |
Introduction
When untreated wastewater containing harmful heavy metals is released into the environment, it causes a slew of health and environmental issues. The most dangerous heavy metals are those that are toxic to human health, such as copper, nickel, and chromium. Heavy metals have an immediate impact on fish and aquatic flowers because they are particularly soluble in liquid settings and are thus easily absorbed by living beings.
People emerge as the very last clients as those species pass up the food chain, and our bodies begin to accumulate massive amounts of heavy metals. Even while heavy metals are occasionally necessary for human health, eating them at excessive levels can have serious consequences for our health. For example, if consumed in large quantities, arsenic is a toxic detail that can cause cancer, organ damage, stunted growth, or even death. Heavy metals such as lead or mercury can trigger autoimmunity, a condition in which the body’s immune system destroys its own cells.
Ion exchange is a chemical process in which free-floating ions of a solid, known as the ion exchanger, exchange positions in solution with other ions of similar charge. The exchanger must have a network-like structure that transports ions and allows them to pass through it, which can be organic or inorganic.
Because of their selectivity against alkali and alkali earth metal cations, chelating ion exchange resins are widely utilized in the recovery and elimination of transition metal ions from industrial solutions. Despite the fact that various artificial1 ion exchanger resins have been the subject of extensive research in recent years due to their selectivity and interaction.
The new guar gum2 based resin with diverse acidic functional groups was synthesized using a modified Porath’s method of polysaccharide fictionalization. The modified guar gum polysaccharide resins were extremely useful and suited for removing heavy hazardous metal ions from industrial waste water. elimination of metal ions such as Zn (II), Cu (II), and Cd (II). Pb (II), Fe (II), Pb(II), Cd(II), and Cr(III) by GAIPA resin, which is now considered one of the most efficient and promising techniques due to its eco-friendly nature, low cost, and speed, because most resins prepared by di-viny benzene styrene backbone and petrochemicals are hydrophobic, expensive, and not as eco-friendly as they were.
Natural polysaccharide resins have a hydrophilic backbone and are inexpensive. Polysaccharide-based cation ion exchange resins are more effective, efficient, and compatible for metal ion separation and concentration in solution, metal ion recovery from waste from the industrial, mineral, textile, and metallurgical industries.
Object of the research work
The goal of this research is to create such compounds, preferably using raw materials sourced locally from industrial or agricultural resources. Among natural polymers, cellulose is of particular interest due to its ease of availability and broad application spectrum in both natural and modified forms.
Methodology
I created polysaccharide matrix-based ion exchangers with various functional groups included. Guar gum provides a hydrophilic foundation for the formulation of chelating resins and is effective and compatible in the separation of metal ions from solution and industrial effluent. GAIPA resin was used in the steel industry to remove metal ions from reference solutions.3
Some fundamental issues arise in the manufacture of guar gum derivatives or polysaccharide derivatives. Because of the abundance of crystalline and amorphous zones in guar gum, a precise treatment with sodium hydroxide is required beforehand. Non-uniformity in NaOH distribution can cause serious issues with the product’s quality and control of properties. These reactions take place at atmospheric pressure.
Because guar gum is a great insulator and these reactions are exothermic, appropriate heat transmission is given in the process design to minimize run-away reactions and hot spots that cause quality deterioration.4
Natural polysaccharides guar gum 2-amino 3-(4-imidazolyl) propanoic acid (GAIPA) resin has been developed and is used for heavy metal ion removal from industrial waste water.
Epichlorohydrin was utilized to etherify guar gum, and these derivatives were subsequently treated with polyamines. Epichlorohydrin is a colourless, mobile liquid with an unpleasant chloroform odour.
Synthesis process of Guar gum 2-amino-3(4-imidazolyl) propanoic acid (GAIPA) resin
In order to make GAIPA resin based on guar gum polysaccharide matrix, 15.51 gm (0.1mole) of 2-amino-3(4-imidazolyl) propanoic acid was dissolved in a 250 ml R.B (round bottom) flask in a minimum amount of methanol. The aforementioned solution was then treated with 9.25gm (0.1 mole) of epichlorohydrin, and the reaction mixture was agitated for 4 hours on a magnetic stirrer. Following that, 4 gm of sodium hydroxide (0.1mole) diluted in a little amount of water was added to the reaction mixture, which was then agitated for 5 hours on a magnetic stirrer and left overnight.
In the second phase of the synthesis, 81 gm of guar gum powder (0.5 mole) was mixed with dioxane in an R.B (round bottom) flask, and 4 gm (0.1mole) NaOH solution was added and swirled on magnetic stirrer at 65°C.
Following that, the reaction mixture from the first stage was added, and the mixture was agitated constantly for 4.5 hours at 65°C. Following a methanol wash, a few drops of HCl are added to eliminate any leftover inorganic impurities and to neutralize any excess alkali. It was finally rinsed with pure methanol.
The guar gum 6-amino-3(4-imidazolyl) propanoic acid (GAIPA) resin was filtered and dried. Finally, the dried resin was weighed on a balance machine and the yield was recorded. The total yield of GAIPA resin was 114.8gm. Figure 1 displays the GAIPA reaction process.
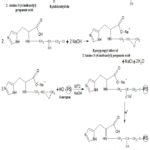 |
Figure 1: Synthesis of Guargam-2-Amino-3-(4-imidazolyl) propanoic acid (GAIPA) resin |
FTIR Characterization of Guar gum 2-amino 3-(4-imidazolyl) propanoic Acid (GAIPA) Resin
The FTIR spectra of GAIPA resin was perfomerd by Kbr disc method. The peak observed at 3671. cm-1 due to –COOH group present in polysaccharide . The peak at the region 3250.2 cm-1 attributed to the –O-H bond stretching. The peak at the region 2913.8 cm-1 is attributed to the N-H bond stretching, which shows the presence of amine group in the structure. The peaks were obtained at 1576.76 cm-1 and 1364.2 cm-1 due to C-H bond stretching. A sharp peak obtained at the region of FTIR 1013.8 cm-1 was due to C-N bond stretching in the structure. FTIR of GAIPA resin was presented in fig no.2.
 |
Figure 2: FTIR of Guar gum 2-amino 3-(4-imidazolyl) propanoic Acid (GAIPA) Resin. |
Ion exchange capacity
The following procedure was performed to compute the overall ion exchange capacity of the resin that was generated. The resin’s capacity was determined using a back titration process. In an Erlenmeyer flask, one gram of resin was inserted, and 200 ml of standardized NaOH (0.05N) and 5 ml of 5% NaCI solution were added. The mixture was then allowed to stand overnight. Using phenolphthalein as an indicator, a 25 ml aliquot of the leftover solutions was back-titrated with a standard standard solution of 0.05 N HCI. To compute total ion exchange capacity, use the equation below5. [Q (Meq/gm)]:
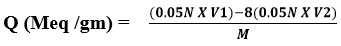
V1 = Volume of 0.05N NaOH (ml)
V2 = Volume of 0.05N HCl (ml)
M = Weight of dry resin (gm)
Q (Meq/gm) = (0.05x 200)-8(0.05x 10.22) /0.88
= (10-8(0.506) / 0.88
= (10-4.04) / 0.88
= 5.96/0.88
= 6.77
Results and discussions
Determination of kd value of metal ions on a GAIPA resin
The batch method was used to determine the Kd of metal ions in resin. In each case, 50 ml of specimen solution was used for Kd analysis, and the pH was adjusted with ammonium salts (hydroxide and chloride). By adding 50 mili gm of GAIPA resin or swirling the liquid for two hours on a magnetic stirrer, the solution’s components were equilibrated. To remove the solution, Whatman filter paper no. 40 was utilized. The remaining part of the filter paper was then equilibrated with 4N HCl, and the liquid was filtered using specialized Whatman filter paper.6,7 The amount of hazardous metal ions in the filtrate and residue were calculated using an AA spectrophotometer. The calibration curves for distinct metal ions were plotted using AAS to analyze many different reference solutions of metal ions. The particular resonance line with varied wavelengths and an oxidizing flame were utilized to assess various metals. The calibration curves were used to measure the amount of metal ion present in the filtrate, and the formula below was used to calculate the percentage of heavy cations removed, as well as the distributor coefficient (Kd) on GAIPA adsorbate.
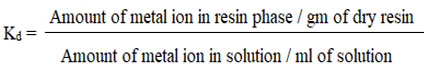
Measurement of removal % of metal ion concentration
After attaining equilibrium with the resin, an AAS spectrophotometer was used to determine the quantity of early metal ions in the solution and filtrate. The percentage of metal ions eliminated was calculated using this formula.
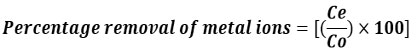
Where Ce constitutes the stable final metal ions concentration in resin and Co represents the initial concentration of metal ions.
Determination of Kd by Batch Method for Steel Industry, Jodhpur (Raj.)
Table 1: Distribution coefficient (Kd) of various Metal ions on GAIPA resin.
|
pH |
Distribution Coefficient of Metal ions on GAIPA resin (Kd X 102) |
|||||||
|
|
Zn (ll) |
Cu (ll) |
Cd (ll) |
Fe (ll) |
Pb (ll) |
Cr(III) |
Ni(II) |
Co(II) |
|
2 |
8.98 |
12.32 |
12.85 |
15.44 |
22.16 |
8.32 |
7.78 |
17.45 |
|
3 |
9.78 |
23.89 |
21.09 |
24.33 |
30.21 |
9.45 |
12.87 |
20.29 |
|
4 |
28.78 |
30.11 |
23.19 |
26.77 |
43.55 |
11.88 |
18.90 |
22.43 |
|
5 |
40.38 |
32.99 |
34.56 |
36.22 |
65.24 |
23.77 |
23.55 |
25.98 |
|
6 |
34.77 |
44.67 |
59.05 |
54.28 |
38.02 |
21.89 |
25.89 |
34.89 |
|
7 |
25.99 |
31.78 |
40.86 |
37.81 |
29.34 |
17.34 |
30.99 |
19.90 |
|
8 |
14.55 |
25.78 |
32.18 |
21.11 |
18.26 |
10.77 |
23.11 |
16.45 |
Table 2: Highest Kd X 102 values of metal ions on GAIPA resin.
|
pH |
Metal ions |
Highest Kd X 102 |
|
5 |
Zn(II) |
40.38 |
|
6 |
Cu(II) |
44.67 |
|
6 |
Cd(II) |
59.05 |
|
6 |
Fe(II) |
54.28 |
|
5 |
Pb(II) |
65.24 |
|
5 |
Cr(III) |
23.77 |
|
7 |
Co(II) |
30.99 |
|
6 |
Ni(II) |
34.89 |
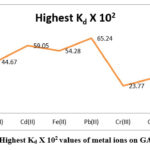 |
Figure 3: Highest Kd X 102 values of metal ions on GAIPA resin. |
The sequence of maximum adsorption rate of metal ions from references solutions on GAIPA resin were follow as Pb(II)>Cd(II)>Fe(II)>Cu(II)>Zn(II)>Ni(II)>Co(II)>Cr(III).
Characteristics of effluents contaminated with heavy metal ions obtained from Steel Industries, Jodhpur (Raj.
Table 3: Characteristics of effluents contaminated with heavy metal ions obtained from Supreme Steel Industries, Jodhpur (Raj.).
|
Color |
Dirty green |
|
pH |
3.1 |
|
Total hardness |
1278 |
|
Metal ion concentration (ppm) |
|
|
Lead |
0.59 |
|
Zinc |
6.10 |
|
Copper |
0.70 |
|
Iron |
3.14 |
|
Cadmium |
0.19 |
|
Calcium |
179.5 |
|
Magnesium |
88.8 |
Smelter, mine, and other nonferrous metal industries’ effluents offer a severe difficulty in the removal of heavy metal ions such as copper, lead, zinc, cadmium, and iron. These heavy metal ions are detected in excess of the allowable limit in the effluent. Although the lime treatment significantly reduces the concentration of unwanted metal ions, the residual metal ion concentration remains at a level regarded dangerous for release into the stream. As a result, more advanced treatment to remove heavy8-12 metal ions from these businesses’ effluent is required. Cation exchangers can successfully lower harmful metal ion concentrations to a predetermined safe level.
The Batch technique was used to determine the distribution coefficient Kd of metal ions on resins. In all cases, 100 ml of sample solution was placed in a conical flask for Kd determination, and the pH was adjusted with suitable buffer. Seventy milligrams of resin have been added to the solution and swirled on a magnetic stirrer for one hour to equilibrate the contents. Whatman filter paper No. 40 was used to filter the solution. The residue on the paper filter was equilibrated with 4N HCl, and the concentration of metal ions13-15 in the filtrate and residue was determined using an atomic absorption spectrophotometer. Calibration curves for various metal ions were plotted after analyzing a series of standard metal ion solutions with AAS.
The calibration curves were used to determine the concentration of metal ions in the filtrate, and the distribution coefficient Kd was derived.
In relation to the above, we synthesized and employed GAIPA for the treatment of Steel Industries wastewater. The Steel Industries in Jodhpur, Rajasthan, provided an effluent sample containing toxic heavy metal ions.
These samples contain heavy metal ions as well as other metal ions, as stated in Table 4.
Table 4: The distribution coefficient (Kd) values of metal ions of Steel Industry on GAIPA resin
|
pH |
Distribution Coefficient of Metal ions on GAIPA resin (Kd X 102) |
||||
|
Zn (ll) |
Cu (ll) |
Cd (ll) |
Fe (ll) |
Pb (ll) |
|
|
3 |
22.09 |
11.27 |
10.89 |
30.11 |
15.70 |
|
4 |
23.91 |
18.21 |
18.62 |
45.33 |
22.10 |
|
5 |
32.87 |
21.91 |
24.04 |
47.89 |
25.93 |
|
6 |
27.96 |
30.35 |
29.78 |
33.93 |
36.07 |
|
7 |
16.39 |
17.07 |
16.94 |
21.70 |
40.90 |
|
8 |
13.70 |
7.91 |
10.56 |
20.06 |
32.77 |
Table 5: Observational analysis of metal ions recovery on a column of Guar gum 2-amino 3-(4-imidazolyl) propanoic acid (GAIPA) resin.
|
Metal ion |
Recovered metal ion% |
Eluent used |
Volume of eluent |
|
Zn(ll) |
92.89 |
0.05N HCl |
50 |
|
Cu (ll) |
91.75 |
2.5N HCl |
50 |
|
Cd (ll) |
90.32 |
0.5N HNO3 |
45 |
|
Fe (ll) |
98.08 |
0.5N HCl |
55 |
|
Pb (ll) |
94.21 |
1.0N HNO3 |
40 |
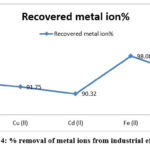 |
Figure 4: % removal of metal ions from industrial effluents |
% removal of metal ions from industrial effluents follow sequence as Fe(II)>Pb(II)>Zn(II)>Cu(II)>Cd(II).
The metal ions were quantitatively eluted with various acid strengths. Pb2+ was eluted with 1 N HNO3; Fe2+ with 0.5 N HCl; Cu2+ with 2.5 N HCl; Cd2+ with 0.5 N HNO3; Co2+ and Ni2+ were eluted with 0.5 N HCl and 1.0 N HCl, respectively. The resin was then extensively rinsed with dematerialized water. It could be reused up to six times with a 96% success rate. Because it is made of biopolymer, the resin may be disposed of by burial or incinerated after elation16, 17.
Conclusions
Finally it was conclude that naturally modified polysaccharide based resin are more efficient and compatible for separation and recovery of metal ions from industrial waste water.
Acknowledgment
We would like to express thanks to my research guide Dr. Aresh Vikram Singh, Professor, Department of Chemistry, Jai Narain Vyas University, Jodhpur, for his excellent guidance, encouragement, motivation and suggestions.
Concflict of interest
The authors declare no conflict of interest in the present work
Funding Sources
There are no funding sources.
References
- Singh, A.V., and Sharma, N.K., “Characterization and applications of synthesized cation exchanges guar gum sulphonic acid (GSA) resin for removal toxic metal ions from industrial waste water”. J. Pretoria, Vol. 37, pp.76, (2011).
CrossRef - Siddiqui, W. A., and Khan, S.A., “Synthesis, characterization and ion exchange properties of Zr (IV) tungstoido-phosphate, a new cation exchanger” Bull .Mater. Sci., Vol 30 (1), pp.43-49.(2007).
CrossRef - Bekar U.G., Guner F.S., Dizman M and Erciyes A.T “Heavy metal removal by ion exchanger based on hydroxyl methyl cellulose” , J.Appl.Poly.Sci. Vol.4, (14) pp.3501-3506.(1999).
CrossRef - Singh,A.V., and Rathore, M., Int. Res. J. of Pure and Appl. Chemistry, Vol.39, pp.216-221, (2023).
CrossRef - Sharma, G., Pathania, D., Naushad, M., Ionics.,Vol.21, pp.1045-1055.(2015).
CrossRef - Singh, A. V., Singh, R., “Environmental Progress & Sustainable Energy., Vol.32(1), pp.103-108.(2013).
CrossRef - Wong K.S., Womg K.H., Nag .S .,Chung, W. K., and Wong, P.K., “Adsorption of copper ion on magnetic immoblized chitin” Water Sci. Tech, Vol.56(14), pp.135-143.(2007).
CrossRef - Singh, A.V., and Sharma, N.K., “GSA resin for removal and recovery of toxic metal ions from industrial waste water” Water SA Journal,Vol.37; pp.295.(2011).
CrossRef - Parihar,N., Rasayan J.Chem. Vol.14 (4), pp.2352-236, (2021).
CrossRef - Gambheer, Geetu, Polimetry, Vol.67 ,pp.(5), (2022)
- Zhang Rui., J. of Ethnopharmology, Vol. 305, pp.89,6april (2023).
- Sujata, Mandal., Hwang, Sangchul., and Sheldon, Int. J. of Boil Macromol, Vol.226, pp.7, dec (2022).
- Caldera, martin., Denis,A., J. of polymer research, Vol.28, pp.10, Oct (2021).
- Nagar,S., Singh,A.V., and Rathore,J.S., Bull. Of Env. Phrm. and Llife Sci, Vol.9, 11, pp.77-88, (2020).
- Nagar,S., “Synthesis of natural polysaccharide based cellulose anthranilic acid ion exchange resin for extrcation of heavy toxic nature ion cadmium from industrial waste water , steel industry , jodhpur by batch method” Research Journal of Chemical Sciences, Vol 12(1), pp.1-10, feb (2022).
- Arora,G., and Gupta,A.P., The Open Access Eng. Journal, Vol.17, pp.65-65,(2023).
CrossRef

This work is licensed under a Creative Commons Attribution 4.0 International License.










