Synthesis, Spectral, Thermal Studies and Antimicrobial Evaluation of Transition Metal Complexes with Novel Schiff Base Ligand
Department of Chemistry, Patkar-Varde College, Goregaon (West), Mumbai, Maharashtra, India.
Corresponding Author E-mail: sopan.chem@gmail.com
DOI : http://dx.doi.org/10.13005/ojc/400118
Article Received on : 04 Dec 2023
Article Accepted on : 06 Jan 2024
Article Published : 18 Jan 2024
Reviewed by: Dr. N Raman
Second Review by: Dr. Vijay H. Masand
Final Approval by: Dr.R.Venkatesh
A novel coumarin Schiff base “(E)-7-hydroxy-4-methyl-8-(1-(1-(naphthalen-2-yl) ethylimino) ethyl)-2H-chromen-2-one” [HOMNEIEC] ligand was synthesized by the condensation of “8-acetyl-7-hydroxy-4-methyl-2H-chromen-2-one” [AHMC] with “1-(naphthalen-1-yl) ethylamine” [NEA]. The transition metal (II) ions complexes with this ligand were synthesized by a common method. The synthesized ligand and metal (II) complexes were studied using spectral (1H NMR, FTIR, Mass Spectrometry) and thermal (TGA) techniques for their structures. The 1H NMR and FTIR spectra of ligand confirm the formation of azomethine bond. The FTIR spectral data validated the formation of coordinate bond through phenolic oxygen on coumarin ring and nitrogen of azomethine with the metal ions. The m/z values in mass spectrum of ligand and its metal complexes were in agreement with their theoretical values of molecular/formula weights. The TGA thermograms suggested there are of two coordinated/lattice H2O molecules in each of the complex. The ligand and metal complexes were evaluated for their in vitro antimicrobial activities using broth microdilution method using DMSO solvent/diluent against Escherichia coli and Pseudomonas aeruginosa a gram-negative bacterial strain, Staphylococcus aureus and Streptococcus pyogenes a gram-positive bacterial strain, and Candida albicans, Aspergillus clavatus and Aspergillus niger a fungal strain. The metal complexes were found to have enhanced antimicrobial activities compare to the Schiff base ligand.
KEYWORDS:Broth Dilution Method; Coumarin Schiff Base; Metal Complexes; Spectroscopy; Thermogravimetry
Download this article as:| Copy the following to cite this article: Adhao S. T, Wagh R. R. Synthesis, Spectral, Thermal Studies and Antimicrobial Evaluation of Transition Metal Complexes with Novel Schiff Base Ligand. Orient J Chem 2024;40(1). |
| Copy the following to cite this URL: Adhao S. T, Wagh R. R. Synthesis, Spectral, Thermal Studies and Antimicrobial Evaluation of Transition Metal Complexes with Novel Schiff Base Ligand. Orient J Chem 2024;40(1). Available from: https://bit.ly/3TZFJgK |
Introduction
The oxygen and nitrogen donor (O, N donor) ligands have a crucial role in the formation of transition metal coordination complexes. A good deal of research was carried out on synthesis, spectral, thermal studies and antimicrobial evaluation of metal complexes with these ligands. The stability and biological activities of these complexes were due to chelating property of the ligand1-7.
In this case the best suitable ligands known are heterocyclic compounds. An important class of organic heterocyclic compounds, acetyl/formyl hydroxy coumarin derivatives and its Schiff bases with aliphatic/aromatic primary amines, have been extensively studied for their structural, biological and photochemical properties. These ligands possess excellent ability to coordinate with the transition metal ions due to formation of chelate through hydroxyl oxygen and azomethine nitrogen8-16.
The metal complexes of coumarin Schiff base ligands with Cu(II), Zn(II), Co(II), and Ni(II) ions will have a better choice for such studies. Since the complexes with these metal (II) ions can be readily prepared17,18 and also play an important role in enhancing their biological activities.
The review of recent year’s literature reveals that Schiff bases of coumarin derivatives with aromatic amines show antibacterial and antifungal19 activities. A broad array of antibacterial activity20 of such Schiff bases have been investigated. However, very few studies were reported about the Schiff base ligands containing both coumarin21-24 and naphthalene23 moieties and its metal (II) complexes. These ligands are polyfunctional molecules consist of oxygen and nitrogen bearing functional groups which can trap the transition metal ions and form metal chelates. Such metal (II) chelates may have higher antimicrobial activities than the Schiff base ligands. This led us to synthesise of new Schiff base ligand containing both coumarin and naphthalene moieties with oxygen and nitrogen donor groups at appropriate positions and its transition metal (II) ion complexes. In this article, synthesis, spectral, thermal studies and antimicrobial evaluation of Schiff base ligand and its bivalent transition metal ions complexes are reported for the first time. This molecule consists of hydroxyl (-OH) and azomethine (>C=N-) groups, which act as a suitable chelating ligand and its metal complexes have moderate to high antimicrobial activities.
Experimental
The reagents, solvents and chemicals used in the experiments were obtained from TCI Chemicals, Loba Chemie, S.D. Fine Chem Ltd. and were utilized as procured. All the solvents required of analytical grade were used and were dried according to the standard procedures in Vogel’s Textbook of Practical Organic Chemistry25. All metal (II) chloride salts were used for metal complexes synthesis. All reactions were stirred magnetically and thin layer chromatography (TLC) Aluminium silica gel coated plates were used to monitored the progress of the reactions and the plates were visualized under Ultraviolet light. The Decomposition points (D.P.) /Melting points (M.P.) were measured by an open capillary method. The metal (II) ions content and metal to ligand ratio of metal complexes were analysed volumetrically by the reported method in Vogel’s Textbook of Quantitative Chemical Analysis26.
FT-IR spectrophotometer in KBr, Bruker Germany, Model No. 3000 Hyperion Microscope with Vertex 80 FTIR System were used to record the IR spectra. NMR spectrometer 600 MHz, JEOL, Japan, ECZR Series, CDCl3 as a solvent and TMS as a reference were used to record 1H NMR spectra. Mass spectra were recorded on a Waters Single COD, Model No. Waters-3100. Perkins Elmer, Model No. TGA-4000 were used to record TGA thermograms. The in vitro antimicrobial evaluation was performed by broth microdilution method and the organisms/strains are listed in Table-3 and Table-4.
Synthesis of 8-acetyl-7-hydroxy-4-methyl-2H-chromen-2-one [AHMC]
Synthesis of “8-acetyl-7-hydroxy-4-methyl-2H-chromen-2-one” [AHMC] was performed from 2, 6-dihydroxyacetophenone [DHAP] and ethyl 3-oxobutanoate [EOB] as per the reported method by Hejchman et al, (2008)27a and Furuta et al, (2004)27b (Scheme-1). Heated a mixture of 2, 6-dihydroxyacetophenone [DHAP] (1.0 mmol) and ethyl acetoacetate [EAA] (1.2 mmol) with catalytic amount of p-toluene sulfonic acid [PTSA] in 10 mL toluene and refluxed for 8 hours with azeotropic removal of water and ethanol by the use of a Dean-Stark trap. The progress of the reaction was monitored using TLC by 50 % Ethyl Acetate/Hexane solvent mixture. The reaction mixture was left overnight. Next day ice-water was added to the mixture. The mixture was stirred for 45 minutes, filtered of and dried the precipitate. Product obtained was purified by recrystallization in hot methanol. Appearance off white and melting point 170 – 172 oC. Yield of the reaction was about 50 % with respect to 2, 6-dihydroxyacetophenone.
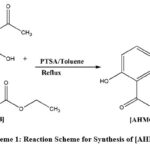 |
Scheme 1: Reaction Scheme for Synthesis of [AHMC]. |
Synthesis of Schiff base (E)-7-hydroxy-4-methyl-8-(1-(1-(naphthalen-2-yl) ethylimino) ethyl)-2H-chromen-2-one [HOMNEIEC] Ligand
Synthesis of a novel Schiff base (E)-7-hydroxy-4-methyl-8-(1-(1-(naphthalen-2-yl) ethylimino) ethyl)-2H-chromen-2-one [HOMNEIEC] ligand was achieved by condensation of “8-acetyl-7-hydroxy-4-methyl-2H-chromen-2-one” [AHMC] and “1-(naphthalen-1-yl) ethylamine” [NEA] as per the method by Aazam et al, (2012)10 (Scheme-2). A clear solution of (S)-1-(naphalen-1-yl) ethane amine [NEA] (1.1 mmol) in 5 mL ethanol was added to a warm solution of 8-acetyl-7-hydroxy-4-methyl-2H-chromen-2-one [AHMC] (1.0 mmol) in 10 mL ethanol with catalytic amount of glacial acetic acid. The resulting mixture was refluxed for 2-3 hours and progress of the reaction was monitored using TLC by 50 % Ethyl Acetate/Hexane solvent combination. The refluxed mixture then kept overnight. Yellow product was precipitated, filtered of and washed with cold ethanol. Product was purified by recrystallization from ethanol, dried in a vacuum desiccator. Appearance yellow colour solid and melting 206 – 208 oC. Soluble in DMF, DMSO and Chloroform. Yield of the reaction was about 60 % with respect to 8-acetyl-7-hydroxy-4-methyl-2H-chromen-2-one.
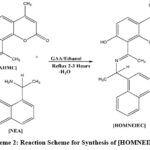 |
Scheme 2: Reaction Scheme for Synthesis of [HOMNEIEC]. |
1H NMR Spectrum of Schiff Base
(δ ppm, CDCl3/TMS) 2.37 (s, 3H, Ar-CH3) a singlet for methyl group attached to coumarin ring at 4th position, 1.90 (d, 3H, >CH-CH3) a doublet for methyl group due to adjacent carbon bearing one hydrogen atom, 1.61 (q, 1H, >CH-CH3) this -CH- give quartet due to three hydrogens on adjacent carbon, 2.68 (s, 3H, -N=C-CH3) methyl group attached to azomethine carbon give singlet, (Figure-1a), 5.95 (s, 1H, Ar-OH) hydroxyl group on coumarin ring at 7th position, 7.26 (s, 1H, =C-H) aromatic hydrogen at 3rd position on coumarin moiety, and 7.90-7.95 (d, 1H), 8.05-8.10 (d, 1H) are two aromatic hydrogens on coumarin ring at 5th and 6th positions respectively and 7.45-7.65 (m, 2H), 7.80-7.84 (m, 5H), all of these multiplet are for hydrogens of naphthalene moiety (Figure-1b).
FTIR Spectrum of Schiff Base
ν (KBr, cm-1): 1730 (lactonyl, -O-C=O) this IR peak is for cyclic ester functional group which is the fundamental part of coumarin, 1592 and 1627 peaks (azomethine, >C=N-) for Schiff base functional group in resonance with the pi electrons of aromatic rings, 1372, 1434, 1508 (aromatic, -C=C-) for aromatic rings, 1238 ( -C-O-) this peak represents -C-O- bond between carbon of coumarin ring and oxygen of hydroxyl group, 1175 (-C-N-) assigned to the carbon of naphthalene ring and nitrogen of azomethine, 1085 (-C-C-) carbon-carbon single bonds, 3432 (hydroxyl, -O-H) a prominent peak in this region of IR spectrum observed for hydroxyl stretching vibration, 2978 (aliphatic C-H) stretching band observed due to the three -CH3 and one -CH- groups, 3048 (aromatic C-H) there are ten aromatic hydrogens present including both coumarin and naphthalene moieties given this band for C-H stretching, 446-924 (aromatic C-H) bending vibrations (Figure-2).
Mass Spectrum of Schiff Base
m/z: (M+H) = 372.5, 284.7, 451.7, 452.1 (Figure-3). m/z of 372.5 represents the base peak. The molecule (M) with H+ give [M+H] + peak. It formed due to the presence of heteroatoms in the molecule. The calculated molecular weight of ligand was 371.45 g/mol. As per the m/z values in the mass spectrum, m/z = 372.5 which represents (M+H) is in agreement with the calculated value. This supports the molecular weight of ligand is equal to 371.45 g/mol.
 |
Figure 1a: 1HNMR Spectrum of Schiff base [HOMNEIEC]. |
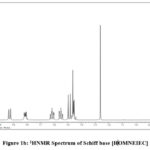 |
Figure 1b: 1HNMR Spectrum of Schiff base [HOMNEIEC]. |
 |
Figure 1c: 1HNMR Spectrum of Schiff base [HOMNEIEC]. |
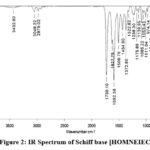 |
Figure 2: IR Spectrum of Schiff base [HOMNEIEC]. |
 |
Figure 3: Mass Spectrum of Schiff base [HOMNEIEC]. |
Synthesis of Metal (II) Complexes
The metal (II) complexes with Schiff base (E)-7-hydroxy-4-methyl-8-(1-((1-(naphthalen-1-yl) ethyl) imino) ethyl)-2H-chromen-2-one [HOMNEIEC] ligand were synthesise by a common method reported by Adhao et al, (in press)34 (Scheme-3). To a clear solution of [HOMNEIEC] (0.5 mmol) in 15 mL dimethylformamide (DMF) was added a respective metal (II) chloride salt (1.0 mmol) separately with catalytic amount of aqueous ammonia (For the purpose to maintain basicity) at 0-5 oC. Then the resulting mixture was moved to room temperature slowly and stirred for 2-3 hours. The progress of the reaction was checked using TLC by 5 % methylene dichloride/methanol solvent mixture. The reaction mixture was diluted with diethyl ether and stirred for 2 hours to obtain the solid product. The obtained solid was filtered on Whatman filter paper and washed with diethyl ether and dried for 30 minutes under vacuum. The yields of the reaction were between 70% to 75% with respect to the Schiff base [HOMNEIEC]. Coloured solid metal complexes were obtained with M.P./D.P. between 180 oC to 190 oC and found soluble in DMF and DMSO.
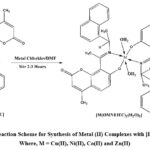 |
Scheme 3: Reaction Scheme for Synthesis of Metal (II) Complexes with [HOMNEIEC] Where, M = Cu(II), Ni(II), Co(II) and Zn(II). |
Table 1: Physical Analysis of Ligand and Metal (II) Complexes
|
Ligand/Complexes |
M.W./F.W. g/mol |
Colour |
% Yield |
M.P./D.P. oC |
At. Wt. of Metal |
% Metal Content Observed (Calculated) |
|
HL2: C24H21NO3 |
371.45 |
Yellow |
60 |
208 |
– |
– |
|
[Co(C24H20NO3)2(H2O)2] |
835.83 |
Black |
70 |
184 |
58.93 |
6.900 (7.050) |
|
[Ni(C24H20NO3)2(H2O)2] |
835.59 |
Brick Red |
75 |
185 |
58.69 |
6.850 (7.024) |
|
[Cu(C24H20NO3)2(H2O)2] |
840.44 |
Sap Green |
72 |
188 |
63.54 |
7.400 (7.560) |
|
[Zn(C24H20NO3)2(H2O)2] |
842.29 |
Buff White |
70 |
190 |
65.39 |
7.600 (7.763) |
M. W. – Molecular Weight, F. W. – Formula Weight, M. P. – Melting Point, D. P. – Decomposition Point, At. Wt. – Atomic Weight
Physical and Spectral Studies of Metal Complexes
Co(II) ion Complex with Schiff Base [HOMNEIEC]: Yield70 %,black colour solid, M.P./D.P. 184 oC, M.W./F.W. 835.83 g/mol, soluble in DMF and DMSO. IR Spectrum: ( ν, cm-1): (Figure-4) 1730, (lactonyl, -O-C=O) cyclic ester part of coumarin ring, 1577 (azomethine, >C=N-) this group frequency shifted to lower side indicate the coordination bond formation with nitrogen, 1398 (aromatic, -C=C-), 1288 (phenolic, -C-O-), 1226 (-C-N-), 1090 (aliphatic, -C-C-), 2900 (aliphatic, -C-H), 3000 (aromatic, C-H), 3155 (Broad) for H2O lattice/coordinated hydrogen bonded, 820 with shoulder peak (coordinated water molecules with metal ion). Mass Spectrum: (Figure-5) m/z: (L+H) = 372.5, this peak is due to ligand. (M+H) = 836.7, 836.9 are m/z for metal complex. The metal complex and H+ combined to give a peak of one unit more than the complex mass. The calculated expected molecular/formula weight of complex was 835.83 g/mol. This is in agreement with the m/z value obtained in the mass spectrum for the metal complex.
Ni(II) ion Complex with Schiff Base [HOMNEIEC]: Yield75 %,brick red colour solid, M.P./D.P. 185 oC, M.W./F.W. 835.59 g/mol, soluble in DMF and DMSO. IR Spectrum: (ν, cm-1): 1721 (lactonyl -O-C=O) cyclic ester, 1593 (azomethine >C=N-) imino, 1391 (aromatic -C=C-), 1282 (phenolic -C-O-), 1218 (-C-N-), 1087 (aliphatic -C-C-), 2900 (aliphatic -C-H stretching), 3000 and 3043 (aromatic -C-H stretching), 3156-3322 (Broad) H2O lattice/coordinated hydrogen bonded, 826 with small shoulder peak (coordinated water molecules with metal ion). Mass Spectrum: m/z (L-H) = 370.4, this peak is for ligand. (M-2) = 833.5, (M-H) = 834.5 are m/z for metal complex. This supports the calculated formula weight for the complex is equal to 835.59 g/mol.
Cu(II) ion Complex with Schiff Base [HOMNEIEC]: Yield72 %,sap green colour solid, M.P./D.P. 188 oC, M.W./F.W. 840.44 g/mol, soluble in DMF and DMSO. IR Spectrum: (ν, cm-1): 1728 (lactonyl -O-C=O) cyclic ester, 1590 (azomethine >C=N-), 1392 (aromatic -C=C-), 1253 (phenolic -C-O-), 1222 (-C-N-), 1086 (aliphatic -C-C-), 2815 (aliphatic -C-H), 3041 (aromatic -C-H), 3223-3323 (Broad) H2O lattice/coordinated hydrogen bonded, 818 & 838 (coordinated water molecules with metal ion). Mass Spectrum: m/z = (L+H) = 372.4, it represents ligand. (M+H) = 841.3 and 841.4 and (M+) = 840.3 are for the metal complex. The peak, m/z = 840.3 is almost equal to the calculated formula weight 840.44 g/mol of the metal complex.
Zn(II) ion Complex with Schiff Base [HOMNEIEC]: Yield70 %, buff whitecolour solid, M.P./D.P. 190 oC, M.W./F.W. 842.29 g/mol, soluble in DMF and DMSO. IR Spectrum: (ν, cm-1): 1730 (lactonyl -O-C=O), 1594 (azomethine >C=N-), 1392 (aromatic -C=C-), 1242 (phenolic -C-O-), 1166 (aliphatic -C-C-), 3000-3100 (aromatic -C-H), 3233-3353 (Broad) H2O lattice/coordinated hydrogen bonded, 663 with small shoulder peak (coordinated water molecules with metal ion). Mass Spectrum: m/z = (L+H) = 372.4, for ligand. (M+H) = 843.2, 843.9, are for the metal complex which is nearly in agreement with the formula weight of the metal complex 842.29 g/mol. Where L is ligand and M is metal complex.
 |
Figure 4: IR Spectrum of Metal (II) Complex with [HOMNEIEC] |
 |
Figure 5: Mass Spectrum of Co(II) Complex with [HOMNEIEC] |
Thermal (TGA) Studies of Metal Complexes:
The TGA thermograms were recorded and the % weight loss in each step was evaluated with respect to the heating rate of 20 oC/minute between 30 oC to 900 oC. The observed and calculated TGA data of % weight loss and residue remained is summarized in Table-2 for the metal complexes of [HOMNEIEC]/HL2 ligand.
Table 2: TGA Data of Metal Complexes
|
Formula of Complexes |
% of Metal ion Observed (Calculated) |
% of H2O Observed (Calculated) |
M.W./F.W. g/mol |
Wt. of Sample (mg) |
% Weight Loss |
||
|
30-105 oC |
105-917 oC |
% Residue (Oxides) |
|||||
|
[Co(C24H20NO3)2(H2O)2] |
7.443 (7.050) |
5.562 (4.307) |
835.83 |
4.765 |
5.562 |
84.700 |
10.138 |
|
[Ni(C24H20NO3)2(H2O)2] |
7.767 (7.024) |
5.897 (4.308) |
835.59 |
3.708 |
5.897 |
84.218 |
9.885 |
|
[Cu(C24H20NO3)2(H2O)2] |
6.795 (7.560) |
6.098 (4.283) |
840.44 |
3.742 |
6.098 |
84.540 |
9.362 |
|
[Zn(C24H20NO3)2(H2O)2] |
9.172 (7.763) |
5.380 (4.274) |
842.29 |
4.843 |
5.380 |
83.203 |
11.417 |
M.W.- Molecular Weight, F.W.- Formula Weight, Wt.- Weight
 |
Figure 6: TGA Thermogram of Co(II) Complex |
Antimicrobial Activities Studies
The in vitro antimicrobial activities of Schiff base [HOMNEIEC]/HL2 and its transition metal (II) ions complexes were evaluated in DMSO medium/diluent using the broth microdilution method29-31. The MIC (Minimum Inhibitory Concentration) values in microgram per mL for the synthesized coumarin Schiff base ligand and its metal (II) ions complexes were determined against E. coli and P. aeruginosa a gram-negative bacterial strain, S. aureus and S. pyogenes a gram-positive bacterial strain, and C. albicans,A. clavatus and A. niger fungal strains listed in the Table-3 and Table-4 and comparisons in their activities shown in Figure-7 and Figure-8 respectively.Chloramphenicol (antibacterial) and griseofulvin (antifungal) drugs were used as reference for this antimicrobial evaluation.
Antibacterial Activity of Ligand and Metal Complexes
Table 3: MIC Values of Schiff Base [HOMNEIEC]/HL2 and Its Transition Metal Complexes
|
Test Compound/ Complex |
Test Organisms Minimal Inhibitory Concentration [MIC] (µg/mL) |
|||
|
Gram Negative |
Gram Positive |
|||
|
E. COLI (MTCC 443) |
P. AERUGINOSA (MTCC 1688) |
S. AUREUS (MTCC 96) |
S. PYOGENES (MTCC 442) |
|
|
HL2: C24H21NO3 |
100 |
125 |
125 |
100 |
|
[Co(C24H20NO3)2(H2O)2] |
125 |
200 |
125 |
200 |
|
[Ni(C24H20NO3)2(H2O)2] |
100 |
125 |
100 |
200 |
|
[Cu(C24H20NO3)2(H2O)2] |
100 |
62.5 |
125 |
125 |
|
[Zn(C24H20NO3)2(H2O)2] |
62.5 |
100 |
100 |
100 |
|
Chloramphenicol |
50 |
50 |
50 |
50 |
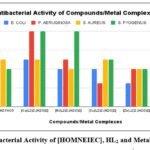 |
Figure 7: Antibacterial Activity of [HOMNEIEC], HL2 and Metal (II) Complexes |
Antifungal Activity of Ligand and Metal Complexes:
Table 4: MIC Values of Schiff Base [HOMNEIEC]/HL2 and Its Transition Metal Complexes
|
Test Compounds/ Complexes |
Test Organisms Minimal Inhibitory Concentration [MIC] (µg/mL) |
||
|
C. ALBICANS (MTCC 227) |
A. CLAVATUS (MTCC 1323) |
A. NIGER (MTCC 282) |
|
|
HL2: C24H21NO3 |
500 |
>1000 |
>1000 |
|
[Co(C24H20NO3)2(H2O)2] |
>1000 |
>1000 |
>1000 |
|
[Ni(C24H20NO3)2(H2O)2] |
500 |
>1000 |
>1000 |
|
[Cu(C24H20NO3)2(H2O)2] |
250 |
1000 |
500 |
|
[Zn(C24H20NO3)2(H2O)2] |
500 |
1000 |
1000 |
|
GRISEOFULVIN |
500 |
100 |
100 |
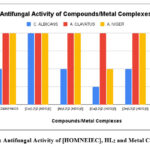 |
Figure 8: Antifungal Activity of [HOMNEIEC], HL2 and Metal Complexes |
Results and Discussion
Synthesis: A novel Schiff Base (E)-7-hydroxy-4-methyl-8-(1-(1-(naphthalen-2-yl) ethylimino) ethyl)-2H-chromen-2-one [HOMNEIEC] ligand was synthesized by condensation of “8-acetyl-7-hydroxy-4-methyl-2H-chromen-2-one” [AHMC] and “1-(naphthalen-1-yl) ethylamine” [NEA] (Scheme-2). “8-acetyl-7-hydroxy-4-methyl-2H-chromen-2-one” [AHMC] was synthesized as per the method reported in the literature (Scheme-1). The metal (II) ions complexes were synthesized by using a common method. The reactions of metal (II) chlorides of cobalt, nickel, copper and zinc separately with the Schiff base ligand were performed to obtained the metal complexes (Scheme-3). The synthesized ligand and its metal (II) ions complexes were studied for their structures by using physical, spectral and thermal methods.
1H NMR Spectroscopy
The characteristic 1H NMR signal for aliphatic -NH2 (between δ 2.0-3.0 ppm)33 does not appeared in the 1H NMR spectrum of ligand proving that the azomethine (>C=N-) bond successfully formed in place of amino (-NH2). The signals for hydrogens of three -CH3 groups, one >CH- group, one phenolic hydrogen and ten aromatic hydrogens were observed with their signature chemical shift values (δ in ppm) that justified the proposed structure of the Schiff base ligand.
FTIR Spectroscopy
The IR spectrum of [HOMNEIEC] consists of various bandsrepresenting -O-C=O (ester), >C=N- (azomethine), -C=C- (aromatic), -C-O-, -C-C-, -O-H (hydroxyl), -C-H (aliphatic C-H stretching), -C=C-H (aromatic C-H stretching), M-N (metal and nitrogen), M-O (metal and oxygen), and C-H bending vibrations. In the IR spectrum of ligand, the peak for amino (-NH2) group does not appear indicating the formation of azomethine (>C=N-) group (Figure-2). The presence of characteristic metal-oxygen and metal-nitrogen IR bands (400-600 cm-1)10, 35 indicate the formation of metal chelates and the ligand is oxygen and nitrogen donor. The characteristic peak for the hydroxyl (-O-H) group does not appear in the IR spectrum of metal complexes. It confirms that the hydrogen atom detached and metal ligand coordinate bond formation takes place through oxygen. The broad peak between 3200-3600 cm-1 suggests presence of lattice/coordinated H2O molecules associated with the metal (II) ions complexes. The peaks between 820-840 indicate the coordinated H2O molecules with the metal ions (Figure-4).
Mass Spectrometry
The m/z values in the mass spectra of ligand and its metal complexes were found in concordance with the calculated values of molecular weight of ligand and the formula weights of metal complexes. The m/z values obtained support the molecular weight of Schiff base (371.45 g/mol) and the molecular/formula weights of metal complexes (Table-1).
Thermogravimetry
The TGA studies showed that the percentage (%) of metal ions observed and calculated were found to be nearly equal (Table-2). The TGA thermograms of the metal complexes suggests that there are coordinated/lattice H2O molecules associated with the metal complexes. The slightly higher observed percentage weight loss in the beginning may be due to the removal of non-coordinated part of ligand such as methyl group28 along with water. It can be seen in the TGA thermograms that the decomposition of metal complexes started between 180 oC to 200 oC and the major loss in weight was observed between 400 oC to 500 oC. For Co (II) and Cu (II) ions complexes decomposition started from 180 oC onwards and sharp loss in weight was observed at around 400 oC and for Ni (II) and Zn (II) ions complexes the decomposition commenced from 200 oC onwards and it was continued upto 500 oC. From the decomposition pattern it is evident that the metal complexes are thermally stable at room temperature. All complexes more or less showed similar decomposition trends. First there was loss of water molecules between 30 oC to 105 oC, decomposition started at 180 oC or 200 oC and a major loss in weight was observed after 400 oC or 500 oC and the decomposition completed at and above 780 oC.
In vitro Antibacterial and Antifungal Evaluation
In vitro antibacterial evaluation (Figure-7) and in vitro antifungal evaluation (Figure-8) of the synthesized coumarin Schiff base [HOMNEIEC]/HL2 and its metal (II) complexes show that the complexes are more active towards the microbes compare to the ligand. They have more toxicity toward the selected microbes than the ligand (Table-3 and Table-4). The MIC value for the Cu(II) ion complex against P. aeruginosa a gram negative bacterial strain was 62.5 µg/mL and the MIC value for the Zn(II) ion complex against E. coli a gram negative bacterial strain was 62.5 µg/mL. These values are nearer to the MIC values for the standard drug chloramphenicol (50 µg/mL). Chloramphenicol is a known antibiotic used in human medicine for treatment of infections due to methicillin sensitive S. aureus a gram-positive bacterial strain. The MIC value for Cu(II) ion complex against C. albicans a fungal strain was 250 µg/mL, which is lower than the MIC value for the standard drug Griseofulvin (500 µg/mL). The antifungal activity of Cu(II) ion complex was found to be two-fold more than Griseofulvin against C. albicans fungal strain. Griseofulvin is an antifungal agent used to treat fungal infections of the skin, hair, and nails. It acts by preventing the growth of fungi.
Conclusion
A bidentate (O, N- donor) Schiff base ligand [HOMNEIEC] containing both coumarin and naphthalene moieties and its metal (II) ions complexes, [M(OMNEIEC)2(H2O)2] were synthesized successfully. The FTIR, 1H NMR, Mass spectrometry, and TGA methods were used to confirm the structures of synthesized ligand (Scheme-2) and its metal (II) ions complexes (Scheme-3). The ligand was found coordinated through phenolic-oxygen (on coumarin ring) and nitrogen of azomethine group forming stable chelates with metal to ligand ratio 1:2 and the metal complexes were found stable at ambient temperature. All of these studies and evaluation concluded that this oxygen and nitrogen donor Schiff base ligand and the selected metal (II) ions have formed stable metal complexes and the Copper (II) and Zinc (II) ions complexes found to have enhanced antimicrobial activities than the ligand.
Acknowledgement
The Authors express their sincere thanks to the Principal, Patkar-Varde College, Goregaon (West), Mumbai for laboratory facilities. Authors are also thankful to SAIF, IIT Bombay, Mumbai and ALR Labs, Hyderabad for instrumentation facilities. Thanks, are also to Microcare Laboratory, Surat for antimicrobial activities studies facilities.
Conflict of Interest
The Authors declares they have no conflict of interest.
References
- Revankar, V. K.; Sathisa, M. P.; Shetti, U. N.; Pai, K. S. R. Eur. J. of Med. Chem. 2008, 43, 2338-2346; https://doi.org/10.1016/j.ejmech.2007.10.003
CrossRef - Chondhekar, T. K.; Munde, A. S.; Jagdale, A. N.; Jadhav, S. M. J. of Ser. Chem. Soc. 2010, 75(3), 349-359; https://doi.org/10.2298/JSC090408009M
CrossRef - Nagawade, A.V.; Dhokale, N. T.; Karale, B. K. Int. J. of Chem. Sci. & Res. 2017, 15(2), 138-146.
- Kudrat-E-Zahan, M.; Hossain, H.; Roy, P. K.; Zakaria, C. M. Int. J. of Chem. Stud. 2018, 6(1), 19-31.
- Yaman, P. K.; Subasi, E.; Temel, H.; Oter, O.; Celik, E. Asi. J. of Chem. 2014, 26(12), 3581-3587; http://dx.doi.org/10.14233/ajchem.2014.16505
CrossRef - Aswar, A. S.; Yaul, S. R.; Yaul, A. R.; Pethe, G. B. Am.-Eur. J. of Sci. Res. 2009, 4(4), 229-234.
- Dalia, S. A.; Afsan, F.; Hossain, S.; Mannan, A.; Haque, M. M.; Kudrat-E-Zahan, M. Asi. J. of Chem. Sci. 2018, 4(4), 1-11; http://dx.doi.org/10.9734/ajocs/2018/42355
CrossRef - Mustafa, B.; Piskin, M.; Durmus, M. J. of Photochem. & Photobio. A: Chem. 2011, 223, 37-49; https://doi.org/10.1016/j.jphotochem. 2011.07.014
CrossRef - Anantha Lakshmi, P. V.; Tyaga, V. J.; Shyamala, B. S. Int. J. of Pharma. and Pharma. Sci. 2010, 2(4), 150-152.
- Aazam, E. S.; Al-Amri, H. M.; EL Husseiny, A. F. Ara. J. of Chem. 2012, 5, 45-53; https://doi.org/10.1016/j.arabjc.2010.07.022
CrossRef - Khanum, S. A.; Prashanth, T.; Vijay Avin, B. R.; Thirusangu, P.; Ranganatha, V. L.; Prabhakar, B. T.; Chandra, J. N. Biomed. & Pharma. 2019, 112, 1-11.
CrossRef - Molnar, M.; Loncaric, M.; Gaso-Sokac, D.; Jokic, S. Biomolecules. 2020, 10 (151), 1-35; https://doi.org/10.3390/biom10010151
CrossRef - Patel, J.; Dholariya, H.; Patel, K.; Bhatt J.; Patel, K. Med. Chem. Res. 2014, 23, 3714-3724.
CrossRef - Vyas, K. B.; Nimavat, K. S.; Jani, G. R.; Hathi, M. V. Int. J. Chem. Sci. 2008, 6(4), 2028-2037.
CrossRef - Hathi, M. V.; Vyas, K. B.; Jani, G. R. E-J. of Chem. 2009, 6(4), 1121-1124; http://dx.doi.org/10.1155/2009/146906
CrossRef - Abid, K. K.; Abbas, B. F. Res. Chem. Intermed. 2013, 39, 3991-3999; http://dx.doi.org/10.1007/s11164-012-0914-1
CrossRef - Aazam, E.S.; EL Husseiny, A. F.; Hitochcock, P. B.; Alshehri, J. M. Cent. Eur. J. of Chem. 2008, 6(2), 319-323; http://dx.doi.org/10.2478/s11532-008-0018-3
CrossRef - Mutalik, V.; Phaniband, M. A. J. of Chem. Pharm. Res. 2011, 3 (2), 313-330.
- Behrami, A. Int. J. of App. Chem. 2019, 6(2), 50-52; https://doi.org/10.14445/23939133/IJAC-V6I2P108
CrossRef - , A. F.; Aazam, E. S.; Al-Amri, H. M. Spectrochimica Acta Part A: Mol. and Biomol. Spectro. 2014, 128, 852-863; http://dx.doi.org/10.1016/ j.saa.2014.03.003
CrossRef - , N. G.; Mathada, M. B. H. J. of Mol. Stru. 2020, 1220, 128659; https://doi.org/10.1016/j.molstruc.2020.128659
CrossRef - Aazam, E. S. JKAU. 2010, 22(2), 101-116; http://dx.doi.org/10.4197/sci.22-2.8
CrossRef - Siddappa, K.; Mallikarjun, K.; Reddy, T.; Mallikarjun, M.; Readdy, C. V.; Tambe, M. E-J. of Chem. 2009, 6(3), 615-624; https://doi.org/10.1155/2009/416836
CrossRef - Furniss, B. S.; Hannaford, A. J.; Smith, P. W. G.; Tatchell, A. R. 1989, Vogel’s Textbook of Practical Organic Chemistry. 5th Ed., Longmans, London.
- Jeffery, G. H.; Bassett, J.; Mendham, J.; Denney, R. C. 1989, Vogel’s Textbook of Quantitative Chemical Analysis. 5th Ed., Longman Scientific & Technical, New York.
- Hejchman, E.; Maciejewska, D.; Wolska, I. Monatch Chem. 2008, 139, 1337-1348; http://dx.doi.org/10.1007/s00706-008-0931-3
CrossRef - Furuta, T.; Takeuchi, H.; Isozaki, M.; Takahashi, Y.; Kanehara, M.; Suigimoto, M.; Watanabe, T.; Noguchi, K.; Dore, TM.; Kurahashi, T.; Iwamura, M.; Tsien, RY. Chem. Bio. Chem. 2004, 5, 1119.
CrossRef - Ingle, V. S.; Bondar, D. V. Asi. J. of Chem. 2023, 35(2), 327-336; http://dx.doi.org/10.14233/ajchem.2023.24036
CrossRef - Isenberg, H. D. Clinical Microbiology Procedure Handbook. 2004, 2, 2nd Ed., 5, 5.0.1.
- Albert, B. Manual of Clinical Microbiology. 1991, 5th Ed., ASM Press: Washington, DC, 1173.
- Rattan, A. Antimicrobials in Laboratory Medicine. 2000, BI Churchill Livingstone: India, 85.
- Hancock, R. E. W.; Wiegand, I.; Hilpert, K. Nat. Protoc. 2008, 3, 163-175; https://doi.org/10.1038/nprot.2007.521
CrossRef - Pavia, D. L.; Lampman, G. M.; Kriz, G. S.; Vyvyan, J. R. Introduction to Spectroscopy. 2015, 5th Ed., Cengage Learning, USA.
- Adhao, S.T.; Wagh, R. R. Orient. J. of Chem.. (in press).
- Mishra, A.; Sharma, R.; Shrivastava, B. D. Ind. J. of Pure & App. Phys. 2011, 49, 740-747; http://nopr.niscpr.res.in/handle/123456789/12951

This work is licensed under a Creative Commons Attribution 4.0 International License.










