Simultaneous Estiamtion of Dolutegravir and Rilpivirine and their Impurities Using RP-HPLC
Ramarao Abburi1 , V D N Kumar Abbaraju2
, V D N Kumar Abbaraju2 , Pushpalatha K1
, Pushpalatha K1 , Satya Vani Chinnamaneni3, G. Venkata Rao4
, Satya Vani Chinnamaneni3, G. Venkata Rao4 and M. V. Basaveswara Rao1*
and M. V. Basaveswara Rao1*
1Department of Chemistry, Krishna University, Krishna Dist., Andhra Pradesh, India.
2Department of Environmental Sciences, GITAM Deemed to be University, Viskhapatnam, Andhra Pradesh, India.
3Principal QC Lab Tech, Waters Corporation, Massachusetts, USA.
4Department of Chemistry, SRR and CVR Govt. Degree College, Vijayawada, A.P, India.
Corresponding Author E-mail: vbrmandava@yahoo.com
DOI : http://dx.doi.org/10.13005/ojc/400105
Article Received on : 13 Nov 2023
Article Accepted on : 05 Jan 2024
Article Published : 12 Feb 2024
Reviewed by: Dr. D. Kalyani
Second Review by: Dr. Naresh batham
Final Approval by: Dr. Ioana Stanciu
A very simple, more accurate, and highly precise process is refined to development of two combination drugs Dolutegravir (DUA), Rilpivirine (RPV)in the tablet dosage form. For this development Agilent 100-5C18 column (250mm x 4.6mm).Acetonitrile as 40v/v and Phosphate buffer which is maintained at pH 4.0as 60v/vis passed with the help of column. Flow rate was measure as 0.80ml/min. Column temperature is accompanied as ambient. Upgraded wavelength is 235 nm. Run time is identified as 10 minutes and the volume injected for this analysis is 20 µl. Retention time of DUA and RPV were found to be 2.6 & 3.9min. %RSD of the DUA and RPV were and found to be 0.87, 0.40 as intraday and 0.75 and 0.56 as inter day precision. % Recovery was obtained as 98.59%, 100.7% for DUA and RPV respectively. The Rt values are minimized as well as run time was decreased. Finally, concluded that this proposed and developed process is more simple as well as economical which should be prevalent in regular QC labs and present in the plasma samples.
KEYWORDS:DUA; Precision; RPV; RP-HPLC; Retention time
Download this article as:| Copy the following to cite this article: Abburi R, Abbaraju V. D. K. N, Pushpalatha K, Chinnamaneni S. V, Rao G. V, Rao M. V. B. Simultaneous Estiamtion of Dolutegravir and Rilpivirine and Their Impurities Using RP-HPLC. Orient J Chem 2024;40(1). |
| Copy the following to cite this URL: Abburi R, Abbaraju V. D. K. N, Pushpalatha K, Chinnamaneni S. V, Rao G. V, Rao M. V. B. Simultaneous Estiamtion of Dolutegravir and Rilpivirine and Their Impurities Using RP-HPLC. Orient J Chem 2024;40(1). Available from: https://bit.ly/3unMqil |
Introduction
Dolutegravir (DUA), is available as Tivicay. It is acting as an antiretroviral medication. This can be used along with drugs for treating HIV/AIDS. 1 It also utilized a part of post exposure prophylaxis, for the prevention of infection caused by HIV follows potential exposure. 2 This drug is utilized for the treatment for adults those who are infected with HIV-those who are not taken any treatment or therapy. This drug may also be using by children with age 12 years with body weight 40 kg. 3 The agency named as FDA approved the drugs named as Tivicay and Tivicay PD in healthcare ViiV. 4 Another drug Rilpivirine(RPV), is available as Edurant and Rekambys. This is the drug developed by Tibotec. This drug is also used to treat HIV/AIDS.5,6 It is a second-generation NNRTI consists of larger than capability, long thermo luminescence and lesser other effects whenever those are correlated by NNRTIs.7,8 As per the author for these drugs 9 they are used UPLC-MS/MS bio analytical method. Authors are used simple protein precipitation by using methanol is process for preparing plasma samples of 50.00μL. This method is validated in the range as 9.7–9700, 52–10,470, 9.7–9730, 73–14,680 and 15–3010 ng/mL. Recovery is ≥76%. The samples of plasma are examined in the Long-term stability of patients which is identified in a time interval of 365 days at a temperature as−40 °C at 4 months for etravirine. Run time is 10 min. Chromatographic separation of Dolutegravir and Rilpivirine10 is achieved performed by using INERTSIL ODS C18 (250 × 4.6 mm, 5μm) column. 0.1% OPA and acetonitrile are used as a mobile phase with a composition as 60v/v and 40 v/v. 10 min is the run time. 1.0 mL min-1 is the flow rate which is observed at a temperature of 250°C. Wavelength is 230 nm. Retention time is 3.4 min and 4.3 min. Linearity limit are 0.999 and 0.999. As per the authors 11 Phenomenex C18 (150×4.6mm, 5μm) is used for this measurement. Mobile phase is 0.1% OPA and acetonitrile as 60:40 v/v. 1.0ml/min is the rate of flow. 262nm is the wavelength identified.4.35 min, and 7.73 min are the retention times. Linearity is 10μg/ml to 150μg/ml for the drug named as DTG and 5μg/ml to 75μg/ml to the drug named as RPV. 98 and 102% are the precision and recoveries. Both the drugs DTG as well as RPV drugs were release 98% in a time interval of 120minuteswhich is observed in the body of rat. The parameters are calculated by considering the rules and regulations given by an international agency named as ICH. This process is applied to plasma samples to study in the areas of bioanalytical. INERTSIL ODS12 column (250×4.6mm, 5µm) is used in this analysis. At pH 6.8 phosphate Buffer as well as acetonitrile are used in the ratio of 35v/v and 65v/v) as mobile phase. 1.0 ml/min is rate of flow. 3.285 min, and 4.635 min are retention times. 99.97% and 100.63% is the purity percentage. 3209 is the system suitability, 1.13 is the theoretical plates, and 5210 is the tailing factor. RP-HPLC-PDA with a BBD13 is used Kromasil 150 mm× 4.6 mm, 5 µm C 18. Assay results were 99.19% and 99.09%. The forced degradation values are 5.96, 4.79, 3.27, 2.36, 0.99, and 4.35 and for DTGR is5.67, 4.44, 4.09, 1.81, 0.43, and 4. For these two drugs14 standard solution consists of strength as 100μg/ml and 50μg/ml are noted at 2.427 min and 4.436 min. The purity percentage is 99.22 % w/v and 99.81 % w/v. Calibration plots were linear at 80μg/ml to 120μg/ml and 30μg/ml to 70μg/ml. Recovery percent is 98 – 102 %. Both the values of LODas well are LOQ are 0.0440µg/ml, 0.060µg/ml and 0.134µg/ml, 0.183µg/ml. The absorption maxima15 of drugs are found at 259.20nm and 305.80nm. Both drugs followed the beer lamberts law in the range of 4µg/ml to 24.00µg/ml and 1.00µg/ml to 8.00µg/ml to the drugs named as DOL and RIL
Materials and methods
analytical/HPLC-grade tertiary butyl alcohol from Alfa Aesar. The monobasic Sodium Hydrogen phosphate, Acetonitrile, potassium hydrogen phosphate, acetic acid as analytical reagent (AR) grade and procured from Spectrum Chemicals, Mumbai, India. Both the DOLT and RIL are taken as gift samples. HPLC-grade methanol and acetonitrile were procured from J. T. Baker. Di-potassium hydrogen phosphate and orthophosphoric acid from Merck Life Sciences. Used high-quality purified water (Milli-Q) for all experimental analyses. The chromatographic analysis was conducted using a Waters Alliance 2695 Module from Waters Corporation.
The standard solutions consist of DUA and RPV are passed into chromatographic system as well as run in various systems of solvent. After reviewing literature, various mobile phases in contrasting configuration also various pH values are tried to identify best circumstances to the separation. Finally, it is concluded as both the solutions like acetonitrile and phosphate buffer gives satisfactory results as correlated to remaining phases. Described composition is tested by disparate ratios as well as by the utilization of various rates of flow. Optimal content for mobile phase is utilized as acetonitrile as well as buffer of phosphate which is maintained a pH at a 4.0. Rate of flow is identified at 0.8ml/min. For this analysis authors are used the column as Agilent 100-5C 18 column [250mm x 4.6mm].UV-Detector is utilized to this analysis.
Optimization of Chromatographic Conditions
For this analysis acetonitrile, pH 4 maintained phosphate buffer used as a mobile phase in a volumetric ratio as 40v/v and 60v/v. The flow rate is considered in between 0.5ml/min to 1.5ml/min. Finally the rate of flow is measured at 1.0 mL/min. Upgraded Optimal wavelength is noted at 235 nm. Run time is identified as 10 minutes and the volume injected for this analysis is 20 µl. Retention time of DUA and RPV were found to be 2.6 & 3.9min.
Preparation of solutions
Mobile phase is prepared by using pH as 4 maintained for phosphate buffer is prepared by dissolving 0.5040gm of Na2HPO4 along with 0.301gm of KH2PO4 in water of grade HPLC after that pH is adjusted as 4 by utilizing glacial CH3COOH. For this content required amount of water is added and after that marked to the volume 100.00ml. Finally the contents are thoroughly mixed at a time interval 10 min.
Stock solutions is prepared by taking
Exactly weighed 25.0000mg of DOLT and RIL prevail taken in2 standard flasks with volume 25.00ml. The contents are thoroughly mixed with the help of mobile phase after that the volume is marked by using the same solvent to achieve stock solutions A as DOLT also B as RIL. The strength of each solution is identified as 1000mg/ml of each and every drug. By using these primary stock solutions, 2.50ml of each one is taken as A as well asB.The contents are taken into 10.00ml standard flask also volume is marked with mobile phase for gettingstrength as 250.00µg/mlof each is DUA and RPV.
Pharma Dosage Sample is prepared by using available tablets in the marketare weighed after that subjected to the crushing process. The power which is obtained is equivalent to 80.00mg of DUAas well as 12.50mg of RPVaresubjected to weighing process after that taken into to a 25.00ml standard flask. The obtained contents are subjected to dissolution in a 10.00ml of mobile phase at a time interval of 15min. Finally, the contents are filtered with0.45m membrane filter. Sonication was performed at a time interval of 20min. with the help of defined stock solution working stock solution is prepared.
Experimental
This process is optimized by keeping the aim to develop systematic procedure to both the drugs DOLT and RIL. Acceptable retention times, theoretical plates, as well as very good resolution are noted by acetonitrile 40v/v, pH 4 contained phosphate buffer 60v/v. Agilent100-5 C18 column, P134 [250×4.5 mm] is used for this analysis. 235nm is the wavelength noted.
Results and discussion
System suitability
This parameter is performed with the help of total five injections which contains the solution of 100% strengthfor 250.00µg/ml of DUA and RPV. The total number of theoretical plates (N) which are identified after that computed tailing factors as T are expressed in table 1 and 2. And the related chromatograms are represented in the figure 1.
Table 1: System Suitability Parameters
|
Parameters |
DUA |
RPV |
|
Rtin min |
2.5 |
3.8 |
|
Theoretical plates as N |
12672 |
10499 |
|
Tailing factor as T |
1.29 |
1.14 |
|
Resolution as Rs |
1.8 |
|
 |
Figure 1: Chromatogram of System Suitability, Chromatogram for trial – I and II |
Table 2: Chromatographic trials.
|
Trials |
Arrangement of mobile phase |
Rate of flow in ml/min |
Drug |
Retention Time in min. |
|
I |
Methanol: Water |
1ml/min |
DOLT |
3.19 |
|
RIL |
3.93 |
|||
|
II |
Acetonitrile: Phosphate buffer pH (3.0) |
1ml/min |
DOLT |
1.9 |
|
RIL |
4.7 |
Accuracy
To this parameter various studies are performed with the help of standard accession process. A well-knownamount of pure drug is combined for pre-analyzed sample as well as the contents by this process after that percent recovery is reported. Final results so obtained are given in table 3 and the related chromatograms are represented in the figure 2.
Table 3: At n=3 Accuracy results.
|
Recovery level
|
DUA |
RPV |
||||||
|
Quantity Added as µg/ml |
Quantity Found in µg/m) |
% Recovery |
Quantity Added as µg/m) |
QuantityFound in µg/ml |
% Recovery |
|||
|
Std., |
test |
Std., |
Test |
|||||
|
50% |
100 |
100 |
197.5 |
98.75 |
100 |
100 |
199.2 |
99.6 |
|
100% |
100 |
150 |
245.3 |
98.13 |
100 |
150 |
257.7 |
103 |
|
150% |
100 |
200 |
296.6 |
98.89 |
100 |
200 |
299.3 |
99.76 |
|
Mean recovery |
98.59 |
100.7 |
||||||
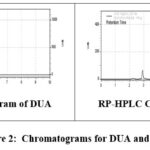 |
Figure 2: Chromatograms for DUA and RPV |
Precision
For this parameter we are documented by calculating %RSD for total 6duplicate injections containing 100 % strength as 250.00mg/ml for DUA and RPV. On the same day also to intermediate precision. % RSD is computed by studies of repeated over various days. The values so obtained are tabulated in the below table 4. And the intra and inter day precision of these two drugs are represented in the figure 3.
Table 4: At n=6 precision results
|
Drug |
Intraday Precision as %RSD |
Interday Precision as %RSD |
|
DUA |
0.79 |
0.88 |
|
RPV |
0.39 |
0.48 |
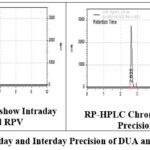 |
Figure 3: Intraday and Interday Precision of DUA and RPV. |
Specificity
This parameter is measured by testing standard materials opposite to the potential intervention. By the experimental analysis it is identified that this process is more specific by utilization of test solution, and there are other interferences are identified. This is mainly because of excipients. The values so obtained are represented as the table 5. The chromatograms for both the drugs named as DUA as well as RPV which are obtained without other interference are shown in the figure 4.
Table 5: Specificity Parameters (n=5)
|
Parameters |
DUA |
RPV |
|
Retention time calculated in min. |
2.9 |
3.8 |
|
Theoretical plates as N |
11796 |
10499 |
|
Tailing factor as T |
1.29 |
1.17 |
|
Resolution as Rs |
1.9 |
|
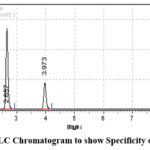 |
Figure 4: RP-HPLC Chromatogram to show Specificity of Sample Solution |
Calibration and Linearity
In order to determine strength of the linearity which is ranged from 50mg/ml to 250mg/ml for both the drugs DUA as well as RPV. For this experimental analysis we are used them, exact aliquots are pipette by working stock solution which is named as A in a series of 10.00ml standard flask. The volume is marked by using suitable solvent. Every solution is passed as n triplicate. curves of calibration are drawn by using the observed peak areas against strength. The calibration curves to both the drugs named as DUA as well as RPV are represented in figure 5 and 6 and their related parameters of linearity are given in the table 2.
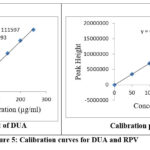 |
Figure 5: Calibration curves for DUA and RPV |
 |
Figure 6: Linearity curves for DUA and RPV |
Table 6: At n=3Linearity results
|
Parameters |
DUA |
RPV |
|
Slope |
184489 |
68818 |
|
y intercept |
111759 |
24921 |
|
Correlation coefficient r2 |
0.9399 |
0.9098 |
|
Regression Equation |
y = 183599x + 111597 |
y = 69917x + 25513 |
|
Linearity range |
50mg/ml to 50mg/ml |
50mg/mlto 250mg/ml |
Robustness
This parameter for this experimental process is documented as altering chromatographic circumstances includes the composition of mobile phase, detection of the wavelength, as well as rate of flow for its authenticity also to know cramped changes in this process circumstances. Solution of high strength by specified variations in operational circumstances are passed to instrument in triplicate. Values so identified for these drugs named as DUA and RPV are reported in the table 7.Chromatogram of Robustness Study for DUA and RPVat a defined Flow rate, wavelength and analyst were represented in the figure 7.
Table 7: Results for Robustness
|
Parameters as n=3 |
%RSD |
|
|
DUA |
RPV |
|
|
233nm wavelength detection |
0.68 |
0.67 |
|
237nm wavelength detection |
0.16 |
0.86 |
|
0.60ml/min as flow rate |
0.28 |
0.97 |
|
1.00ml/min as flow rate |
0.51 |
0.39 |
|
Analyst I |
0.69 |
0.72 |
|
Analyst II |
0.95 |
0.85 |
 |
Figure 7: RP-HPLC Chromatogram of Robustness Study for DUA and RPVat a defined Flow rate, wavelength and analyst |
Detection and Quantitation Limits
These parameters are computed by slopes for plots of calibration as well as the standard deviation noted as SD for areas of the peak. The values are represented in the8. And recovery levels are represented in the figure 8.
Table 8: Detection limit for DUA and RPV
|
Parameters |
DUA |
RPV |
|
LOD |
2.00mg/ml |
1.19mg/ml |
|
LOQ |
6.60mg/ml |
3.92mg/ml |
 |
Figure 8: RP-HPLCChrmoatogram of 50%, 100% and 150% Recovery Level |
Analysis of Marketed Formulations
20.00ml solution of sample with strength as 250 mg/ml for DUAmg/ml and 250 mg/ml of RPV is passed into system of chromatography and responses of the peak are noted. Solution is passed in total three times into column. The quantity of drug which is present as well as the purity percentage is demonstrated by comparing areas of peak standards by samples of testing solution. Aemblematic chromatogram to the assay of marketed formulation are represented in the figure 5 also identified DUA are represented in the table 9.The assayed marketed formulation is represented in the figure 9.
Table 9: At n=3 results for assay in marketed formulation
|
Drug |
Label claim as mg/tab |
Recovered quantity |
% quantity found in drug |
|
DUA |
50 |
49.23 |
98.46% |
|
RPV |
25 |
24.8 |
99.2% |
 |
Figure 9: RP-HPLC Chromatogram of Assay of Marketed formulation |
Conclusion
Throughout the analytical process analytes are stable. For this development Agilent 100-5C18 column (250mm x 4.6mm). Acetonitrile as 40v/v and Phosphate buffer which is maintained at pH 4.0as 60v/v is passed with the help of column. The mobile phase is used for this analysis is so simple for the preparation and economical. Flow rate was measure as 0.80ml/min. Column temperature is accompanied as ambient. Upgraded wavelength is 235 nm. Run time is identified as 10 minutes and the volume injected for this analysis is 20 µl. Retention time of DUA and RPV were found to be 2.6 & 3.9min. %RSD of the DUA and RPV were and found to be 0.87, 0.40 as intraday and 0.75 and 0.56 as inter day precision. % Recovery was obtained as 98.59%, 100.7% for DUA and RPV respectively. The Rt values are minimized as well as run time was decreased. By using the experimental values as well as the parameters, it is finalized as both the drugs DUA as well as RPV are found as ecofriendly, reliable, reproducible simple, precise, accurate along with the high resolution also less retention time which produce this proposed process is more acceptable as well as cost-effective. This method is not a time consuming also this should be utilized clinical studies to analysis of bioavailability as well as bioequivalence
This proposed method is profitably applied to assess in vitro dissolution of DUA as well as RPV which is loaded as a fixed dose consolidation of the tablets. Same method by using equal mobile phase is enforced over plasma of rat plasma and authors are not observed any other interference.
Acknowledgement
The authors are thankful for management of Krishna University for conditional necessary facilities to accomplish this research work.
Conflicts of Interest
There are no conflicts of interest among the authors to perform this work.
Funding Sources
There is no funding sources.
References
- British national formulary : BNF 69 (69 ed.). British Medical Association. 2015. p. 429. ISBN 9780857111562.
- “Dolutegravir Sodium Monograph for Professionals”. Drugs.com. Retrieved 20 April 2019.
- “FDA approves new drug to treat HIV infection”. Food and Drug Administration. 12 August 2013. Archived from the original on 8 February 2014.
- “FDA Approves Drug to Treat Infants and Children with HIV”. U.S. Food and Drug Administration (FDA) (Press release). 12 June 2020. Retrieved 12 June 2020.
- “TMC278 — A new NNRTI”. Tibotec. Archived from the original on 2008-12-20. Retrieved 2010-03-07.
- Stellbrink HJ. “Antiviral drugs in the treatment of AIDS: what is in the pipeline?”. European Journal of Medical Research. 2007; 12 (9): 483–495. .
- Goebel F, Yakovlev A, Pozniak AL, Vinogradova E, Boogaerts G, Hoetelmans R, et al. “Short-term antiviral activity of TMC278–a novel NNRTI–in treatment-naive HIV-1-infected subjects”. AIDS. 2006; 20 (13): 1721–1726. doi:10.1097/01.aids.0000242818.65215.bd.
CrossRef - Pozniak A, Morales-Ramirez J, Mohap L, et al. “48-Week Primary Analysis of Trial TMC278-C204: TMC278 Demonstrates Potent and Sustained Efficacy in ART-naïve Patients. Oral abstract 144LB”. 14th Conference on Retroviruses and Opportunistic Infections. Archived from the original on October 19, 2007.
- Pauline D.J. Bollen, Marga J.A. de Graaff-Teulen, Stein Schalkwijk, Nielka P. van Erp, David M. Burger,Development and validation of an UPLC-MS/MS bioanalytical method for simultaneous quantification of the antiretroviral drugs dolutegravir, elvitegravir, raltegravir, nevirapine and etravirine in human plasma; ,Journal of Chromatography B, 2019; 1105, 76-84, https://doi.org/10.1016/j.jchromb.2018.12.008.
CrossRef - M. Niranjan Babu and R. Chandrasekar; development and validation of stability indicating Rp-HPLC method for the simultaneous estimation of dolutegravir and rilpivirine by forced degradation studies; International Journal of Pharmaceutical Sciences and Research, 2021; 12(9):4954-4963. DOI: http://dx.doi.org/10.13040/IJPSR.0975-8232.12(9).4954-63
CrossRef - B, V., and N. VMK. “Development and validation of Rp-HPLC method for the estimation of dolutegravir and rilpivirine in bulk and pharmaceutical dosage form and its application to rat plasma”. Asian Journal of Pharmaceutical and Clinical Research, 2019; 12(2): 267-71, doi:10.22159/ajpcr.2019.v12i2.2966.
CrossRef - Sivagami B, Sharmil Kumar. L.M, Chandrasekar. R, Niranjan Babu. M. Development and Validation for the Simultaneous Estimation of Rilpivirine and Dolutegravir in Bulk and Pharmaceutical Dosage Forms by RP-HPLC Method. Research Journal of Pharmacy and Technology. 2022; 15(11):5302-6. doi: 10.52711/0974-360X.2022.00893.
CrossRef - Wahab S, Khalid M, Ahmad S, Sweilam SH. Rilpivirine and Dolutegravir Simultaneously Measured via RP-HPLC-PDA with Box–Behnken Design Application: A Study of Forced Degradation under Various Conditions. Separations. 2023;10(3):185. https://doi.org/ 10.3390/ separations10030185.
CrossRef - yusuff, I., Vara Prasad, M., Shaheedha, S. M., & Habeeb, M. (2019). A New Stability Indicating RP-HPLC Method Development and Validation for the Simultaneous Estimation of Dolutegravir and Rilpivirine in Bulk and its Dosage Forms. Iranian Journal of Pharmaceutical Sciences, 15(4), 53-72. doi: 10.22034/ijps.2018.91032.1465
- Sandip T. Thoke, Umesh T. Jadhao, Gunesh N. Dhembre. Development and Validation of UV Spectrophotometric Methods for Simultaneous Estimation of Dolutegravir Sodium and Rilpivirine Hydrochloride in Pure Bulk Form. Asian Journal of Pharmaceutical Analysis. 2022; 12(3):181-6. doi: 10.52711/2231-5675.2022.00031
CrossRef

This work is licensed under a Creative Commons Attribution 4.0 International License.









