Schiff’s Base Imidazole Derivatives Synthesis and Evaluation for their Anti-Inflammatory Activity
1Amity Institute of Pharmacy, Amity University, Noida-201313, Uttar Pradesh, India.
2Sunder Deep Pharmacy College, Dasna, Ghaziabad, Uttar Pradesh, India.
3Glocal School of Pharmacy; Glocal University, Mirza Pur Pole, Saharanpur, Uttar Pradesh, India.
Corresponding Author E-mail: tsdivass7@gmail.com
DOI : http://dx.doi.org/10.13005/ojc/400119
Article Received on : 01 Dec 2023
Article Accepted on : 24 Jan 2024
Article Published : 31 Jan 2024
Reviewed by: Dr. B .K Sharma
Second Review by: Dr. Gaurav
Final Approval by: Dr. Aarif khan
This study is focused on the synthesis and exploration of Schiff’s base imidazole derivatives with the aim of assessing their anti-inflammatory activity. A series of compounds were synthesized and characterized using spectroscopic techniques. In-silico docking analysis was employed to identify potential active ingredients. The anti-inflammatory properties of these derivatives were then investigated using paw edema model induced by carrageenan followed by assessment of TNF-α and IL-1β as inflammatory cytokines. Results showed that specific Schiff’s base Imidazole derivatives, notably C1IN, C2IN, C4IN, C5IN, and C11IN, demonstrate significant effectiveness in alleviating paw edema and reducing the level of IL-1β and TNF-α. The findings emphasize the potential of these developed derivatives as viable options for anti-inflammatory intervention. The observed reduction in paw edema and cytokine levels signifies a promising anti-inflammatory profile, positioning these compounds as candidates for further exploration and development. The study contributes valuable insights into the anti-inflammatory properties of imidazole derivatives, suggesting their potential therapeutic applications in inflammatory conditions. Future research should delve deeper into mechanistic aspects and conduct additional preclinical studies to validate the translational potential of these derivatives in anti-inflammatory pharmacotherapy. This research opens avenues for the development of novel anti-inflammatory agents with potential clinical relevance.
KEYWORDS:Anti-Inflammatory potential; In-Silico Docking analysis; Schiff’s Base imidazole derivatives; Synthesis; Spectroscopy Analysis
Download this article as:| Copy the following to cite this article: Singh D, Kharb R, Sharma S. K. Schiff’s Base Imidazole Derivatives Synthesis and Evaluation for their Anti-Inflammatory Activity. Orient J Chem 2024;40(1). |
| Copy the following to cite this URL: Singh D, Kharb R, Sharma S. K. Schiff’s Base Imidazole Derivatives Synthesis and Evaluation for their Anti-Inflammatory Activity. Orient J Chem 2024;40(1). Available from: https://bit.ly/48U08IJ |
Introduction
Inflammation is a complex biological response that serves as a defense mechanism against harmful stimuli, including pathogens such as microorganisms. The interaction between microorganisms and the host’s immune system plays a pivotal role in the initiation and regulation of inflammation. Understanding the dynamics of microorganism-induced inflammation is crucial for elucidating the pathophysiology of various infectious diseases and developing targeted therapeutic interventions 1. Microorganisms, including bacteria, viruses, fungi, and parasites, have evolved diverse strategies to interact with host tissues and elicit inflammatory responses. The recognition of microorganisms by the host’s immune system involves interleukins 2.
The initial encounter between microorganisms and the host often occurs at mucosal surfaces, where specialized epithelial cells act as a physical barrier. However, pathogens can breach this barrier, leading to the activation of innate immune action. Macrophages, neutrophils, and dendritic cells as innate immune cells play crucial roles in recognizing and eliminating invading microorganisms. Macrophages, for instance, phagocytose pathogens and release cytokines such as tumor necrosis factor-alpha (TNF-α) and interleukin-1 beta (IL-1β), amplifying the inflammatory response 3,4. The adaptive immune system, with its T and B lymphocytes, is subsequently engaged to mount a more specific and targeted defense against the invading microorganisms. T lymphocytes recognize specific antigens presented by infected cells, while B lymphocytes produce antibodies that can neutralize or opsonize pathogens for destruction 5. However, dysregulation of microorganism-induced inflammation can lead to chronic inflammatory conditions and contribute to the pathogenesis of various diseases. Excessive or prolonged activation of immune responses may result in tissue damage, autoimmune disorders, or persistent infections. Conversely, an inadequate immune response may allow microorganisms to evade clearance, leading to chronic infections 6,7.
Nitrogen-based heterocycles, specifically imidazole compounds derived from Schiff bases, have garnered significant attention for their noteworthy role in modulating inflammatory processes. These compounds exhibit diverse pharmacological activities, and their anti-inflammatory potential stems from their ability to interact with various molecular targets involved in the intricate cascade of inflammatory responses 8,9. Imidazole compounds derived from Schiff bases are characterized by a versatile chemical structure, featuring a five-membered ring containing two nitrogen atoms. The presence of nitrogen atoms within the imidazole ring imparts unique chemical reactivity and biological activity to these compounds. Schiff bases, derived from the condensation of an imine and an amine, serve as valuable precursors for the synthesis of imidazole derivatives 10. One of the key mechanisms through which imidazole compounds derived from Schiff bases exert their anti-inflammatory effects is by modulating the activity of enzymes involved in the arachidonic acid metabolism pathway. Inhibition of enzymes, imidazole derivatives help mitigate the inflammatory response and alleviate associated symptoms 11–14.
Moreover, imidazole compounds have been reported to interact with various receptors and transcription factors implicated in inflammation. For instance, they may target and modulate the activity of NF-κB signaling, these compounds can downregulate TNF-α and IL-1β 10,15–17. Additionally, imidazole-based Schiff base derivatives possess antioxidant properties, contributing to their anti-inflammatory effects. Oxidative stress is intricately linked to inflammation, and the ability of these compounds to scavenge reactive oxygen species helps attenuate oxidative damage and curb the inflammatory response 11,12.
The synthesis and exploration of novel imidazole compounds derived from Schiff bases continue to be an active area of research, aiming to unravel their precise molecular mechanisms and optimize their pharmacological properties. The multifaceted roles of these compounds in modulating inflammatory pathways underscore their potential as promising candidates for the development of anti-inflammatory agents with enhanced efficacy and reduced side effects 18. As research progresses, a deeper understanding of the structure-activity relationships and specific molecular targets of these compounds will contribute to their therapeutic applicability in inflammatory conditions.
Material and methods
Chemicals, reagents and software
2,5-dichloroaniline, 4-Fluoroaniline, 3,5-Dimethoxyaniline, 3,5-difluoroaniline, Ethylene Diamine, 2,5-Dibromoaniline, 3,4,5-trimethoxy aniline, Tert-butylamine, 3,5-Bis(Trifluoromethyl)benzyl amine, 2-aminophenol, 3,5-dimethylaniline, 2-amino4,6-dichloropyrimidine, 4-amino-2-hydroxypyrimidine, 2-chloro-6-methylaniline, cyclohexylamine, methoxybenzylamine and 3-aminopyrazole were used in this study and acquired from Sigma-Aldrich and SRL Pvt. Ltd, India. The software used for the in-silico docking analysis was Autodock tools 1.5.7, Discovery studio 2021 and commend prompt used to assess the docking score.
Chemistry for the synthesis
Schiff’s bases were synthesized in two different pathways that are synthesis of imidazole-2-carboxaldehyde and synthesis of various primary amines and Schiff’s Base synthesis.
Synthesis of Imidazole-2-carboxaldehyde
Four-step reaction is involved in this synthesis. The first step involved the synthesis of 1-benzoyl-2-(1,3-dibenzoyl-4-imidazolin-2-yl) imidazole. Using this procedure, 125 mL of acetonitrile, 12 g (0.12 mol) of triethylamine, and 4.25 g (0.06 mol) of imidazole were added to a mouthed, round-bottomed flask. Additionally, 16.8 ml (0.12 mol) of benzoyl chloride was included dropwise to the resultant mixture over the course of a 1-hour period at 15–25°C. Following addition, the stirring procedure was carried out at room temperature for an additional hour. In addition, 625 ml and 125 mL of distilled water and ether were added, and the operating temperature was raised to 5°C. TLC was used for tracking the reaction. After filtering out the crystalline the final product, it was allowed to air dry. After the filter cake was rinsed with water, acetone, and ether in that order, 16g of product with a melting point of 197–198°C was discovered after air drying.
2-(1,3-Dibenzoyl-4-imidazolin-2-yl)imidazole hydrochloride synthesis is the subsequent step. 15 g of dry, unrecrystallized 1-benzoyl-2-(1,3-dibenzoyl-4-imidazolin-2-yl) imidazole, 50 mL of technical-grade methyl alcohol, and 3 mL of concentrated HCl were gradually added to a beaker. The solids gradually dissolved while stirring the mixture. After one hour, an apparent yellow solution form. It is left standing there for an additional five hours, during which time a white solid product precipitate. After that, 150 mL of diethyl ether was added, and the mixture was left overnight. TLC was used to monitor the reaction. The crystals are filtered, rinsed with fresh ether, and then allowed to air dry to yield 10–12 g of the end product at 238–239° C.
3-(1,3-Dibenzoylimidazolidin-2-yl)imidazole hydrochloride synthesis is the third step. 10.0 g of dry, recrystallized 2-(1,3-dibenzoyl-4-imidazolin-2-yl) imidazole hydrochloride dissolved in 30 mL of ethyl alcohol were added to a Parr hydrogenation bottle. Next, for one hour, hydrogenation was carried out on carbon with 0.02 g of 10% palladium present. 95% ethyl alcohol was used to rinse the residue, and the filtrate was then stripped to leave behind solid, impure product. This was rinsed and filtered with fresh acetone, triturated with 200 mL of ice-cold acetone, and finally dried with ether. TLC was used to monitor the reaction. With a melting point of 225–226°C, the final yield of the synthesized compounds was determined to be 8.2–8.8 g. The synthesized compounds were characterized structurally through the interpretation of IR, NMR, and LCMS.
In last step, imidazole-2-carboxaldehyde Synthesis (1A), Eight grams of dry, unrecrystallized 2-(1,3-dibenzoylimidazolidin-2-yl) imidazole hydrochloride were refluxed for ten hours in a solution containing twenty milliliters of concentrated hydrochloric acid, thereafter it was cooled with ice, benzoic acid began to deposit and filtered out. When the filtrate evaporates, a residue is left behind that was first broken down with 10 milliliters of 95% ethyl alcohol and then allowed to cool on ice. Filtered off, the rest of the solids constituted almost pure ethylenediamine dihydrochloride. In order to leave solid residue, the filtrate solvent was once more removed under low pressure and dissolved in 40 milliliters of distilled water. Imidazole-2-carboxaldehyde crystallizes when solid sodium bicarbonate is added and allowed to remain there until the foam stops. TLC was used to monitor the reaction using methanol:hexane (1:5, v/v). By using this method, it was discovered that the compound contained 3.2–3.7 g of crystallized matter, with a melting point of 206–207°C. By interpreting IR, NMR, and LCMS data, compounds that have been produced will be structurally characterized. Figure 1 shows the schematic for the formation of Schiff’s base imidazole derivatives.
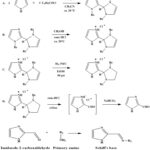 |
Figure 1: Scheme for Schiff’s base Imidazole derivatives synthesis. |
Spectroscopic characterization and in-silico docking analysis
The compounds generated in this investigation underwent identification and characterization through diverse spectroscopic methods, including MS, FT-IR, and NMR. These techniques were applied in accordance with established protocols, with minor modifications as outlined in the relevant references 19,20. Furthermore, an in-silico analysis targeting Tumor Necrosis Factor Alpha (TNF-α; protein grid dimensions center_x, y, z are -3.641, 68.631, 131.508, size_x, y, z are 54, for each) was conducted to discern therapeutically active components. The outcomes are depicted from Figure 2-15. The compounds identified are detailed in Table 1, and the spectroscopic findings of compounds exhibiting therapeutic activity are presented accordingly.
 |
Table 1: Synthesized imidazole derivatives and docking profile with TNF- α. |
Table 2: Spectroscopic characterization of compounds.
|
S. No. |
Compound name (Code) |
Molecular weight |
FTIR characteristic |
NMR |
|
|
1HNMR |
13CNMR |
||||
|
1. |
2-[(1H-imidazol-2-ylmethylidene)amino]phenol (C1IM) |
187.07 |
3425.21, 3350.99 (OH, NH/NH2), 3051.82 (CH), 1678.29 (C=N), 1520.97 (C-O), 1318.06 (CH stretching), 1083.77 |
δ 9.8189 (1H, s), 8.3184 (1H, s), 7.7373 (1H, s), 7.1132 (4H, m), 6.5356 (2H, s) |
δ 164.38 (C=N), 153.27 (C-OH), 137.39 (C-N), 136.25 (C=N), 128.47 and 127.24 (3C), 123.98, 119.19, and 115.23 (3C) |
|
2. |
N-(4-chlorophenyl)-1-(1H-imidazol-2-yl)methanimine (C2IM) |
205.4 |
3589.32, 3408.25, 3272.99 (N=N/NH), 1579.67 (C=N), 1268.99 (C-H), 1039.73 (C-O) |
9.0089 (1H, s), 8.3544 (1H, s), 7.9707 and 7.9508 (1H, d), 7.3240, 7.0143 (2H, d) and 6.9771 (2H, s) |
163.04 (C=N), 114.28 (C-N), 137.52 (C-N), 133.44 (C-Cl), 130.09, 128.21, 123.52 (6C) |
|
3. |
1-(1H-imidazol-2-yl)-N-(3,4,5-trimethoxyphenyl)methanimine (C4IM) |
261.28 |
3618.21, 3531.89 (N=N/NH), 1547.28 (Ar-C=C), 1493.26 (C-H), 1219.99 (C-O), |
8.9724 (1H, s) 7.7325 (1H, s), 6.6443 (1H, s), 6.3013 (4H, s, s), 6.6842, 3.5212 (9H, s, s) |
164.23 (C=N), 152.69 (2C-OCH3), 143.22 (C-N), 138.11 (C- OCH3), 137.52 (C-N), 127.64 (2C), 103.15 (2C), 62.03 and 55.69 (2C-2CH3) |
|
4. |
N-(3,5-dimethoxyphenyl)-1-(1H-imidazol-2-yl)methanimine (C5IM) |
231.26 |
3458.37 (N-N/N=N), 2923.55, 1541.27 (Ar-C=C), 1327.25 (C-O), 1137.23 (C-H) |
8.7102 (1H, s), 7.7359 (1H, s), 6.9659 (2H, s), 6.7128 (3H, s), 3.8114 (6H, s) |
164.13 (C=N), 163.11 (2C-2CH3), 152.69 (2C-N), 137.52, 127.64, 103.15, 99.05 (5CH), 55.69 (2CH- 2CH3) |
|
5. |
5-(hydroxymethyl)-4-[(1H-imidazol-2-ylmethylidene)amino]pyrimidin-2-ol (C10IM) |
219.20 |
3387.89, 3063.21, 1639.83, 1581.34, 1427.71, 1211.93 |
11.4322 (1H, s), 9.1324 (1H, s), 8.5229 (1H, s), 7.6129 (1H, s), 6.5012 (2H, s), 5.3328 (1H, s), 4.5528 (1H, s) |
164.23 (C=N), 158.96 (CN-OH), 157.19 (C=N), 147.22 (C-N), 137.52 (C=N), 127.64 (2CH), 103.15 (1C), 60.03 (C-OH) |
|
6. |
1-(1H-imidazol-2-yl)-N-(4H-1,2,4-triazol-4-yl)methanimine (C11IM) |
162.16 |
3357.39, 3048.25 (NH/N=N), 1549.41 (Ar-C=C), 1342.57 (C-O), 1236.88 (C-H) |
11.1921 (2H, s), 8.3291 (1H, s), 7.9138 (1H, s), 6.5983 (2H, s) |
164.23 (C=N), 145.22 (2CH), 138.11 (C=N), 127.64 (2CH) |
 |
Figure 2: MS spectra of different imidazole components (C1IM and C5IM). |
 |
Figure 3: MS spectra of different imidazole components (C2IM and C10IM). |
 |
Figure 4: MS spectra of different imidazole components (C4IM and C11IM). |
 |
Figure 5: FT-IR spectra of different imidazole components (C1IM and C5IM). |
 |
Figure 6: FT-IR spectra of different imidazole components (C2IM and C10IM). |
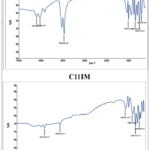 |
Figure 7: FT-IR spectra of different imidazole components (C4IM and C11IM). |
 |
Figure 8: 1HNMR spectra of different imidazole components (C1IM and C5IM). |
 |
Figure 9: 1HNMR spectra of different imidazole components (C2IM and C10IM). |
 |
Figure 10: 1HNMR spectra of different imidazole components (C4IM and C11IM). |
 |
Figure 11: 13 CNMR spectra of different imidazole components (C1IM and C5IM). |
 |
Figure 12: 13 CNMR spectra of different imidazole components (C2IM and C10IM). |
 |
Figure 13: 13 CNMR spectra of different imidazole components (C4IM and C11IM). |
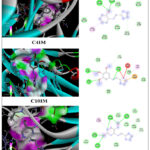 |
Figure 14: Representation of interaction profile of synthesized components (C1IM, C4IM and C10IM) with the TNF-α protein. Based on in- Silico docking study. |
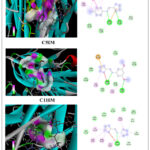 |
Figure 15: Representation of interaction profile of synthesized components (C2IM, C5IM, and C11IM) with the TNF-α protein. Based on in- silico docking study. |
Experimental animals
This study aims to examined the efficacy of synthesized compounds in inflammation induced by carrageenan. The effect of Schiff’s base derivatives was evaluated via measuring the developed paw edema. With prior approval from the Institutional Animal Ethics Committee (163/PO/RE/S/12/CPCSEA/2021-6), Wistar albino rats from institution were used for the in vivo experimental research. The animals in the experiment were kept in polypropylene cages and weighed one at a time, averaging 190 ± 20 g. Following that, they were acclimated to typical laboratory settings, which included a 12:12 h light/dark cycle, at 23 ± 2°C, and a RH 55 ± 5%. The animals were given an ad libitum diet consisting of pellets and saline throughout the trial. The study concluded successfully in compliance with the IAEC and CPCSEA standard guidelines.
There were nine groups in the carrageenan-induced rat paw edema model, and each group had six rats. A 100 μl intraplantar injection of 1% (w/v) lambda carrageenan suspended in saline was used to induce edema. In order to assess the preventive impact against inflammation caused by carrageenan, 300 mg/kg of each drug was received by each group. Using a plethysmometer, the volume of paw edema was examined at pre-and post treatment of carrageenan at intervals of 0 to 300 minutes. The usual medication was indomethacin. After 300 minutes, the animals were put to sleep, and the feet that had developed edema were removed and kept in storage at − 80°C. Following the experiment’s conclusion, the blood from each rat was removed and examined for additional biomarkers associated with inflammation 21 22.
Measurements of pro-inflammatory markers
A modified version of the conventional protocol was used to evaluate the biomarkers in the blood. All animals were remained unconsciousness using ketamine hydrochloride (80–90 mg/kg) after their final paw estimation, and they were then sacrificed so that blood samples could be taken. Using an ELISA kit acquired from Abbkine (China), the blood samples were analysed as per protocol for estimation of TNF-α and IL-1ß 22, 23.
Statistical analysis
To evaluate significant differences, statistical variability was determined as Mean ± SD (n = 6). Statistical analysis involved the Tukey test. The difference between the variables was determined based on the p-value summary, with values considered statistically significant when *p-value < 0.05.
Results
Schiff’s base imidazole derivatives were synthesized as well as characterized using spectroscopic techniques. In-silico docking analysis was employed to identify potential active ingredients. Subsequently, their anti-inflammatory properties were evaluated in the context of paw edema induced by carrageenan in Wistar rats. The study’s findings can be summarized as follows.
Anti-inflammatory activity of the synthesized compounds
Synthesized molecules demonstrated productive anti-inflammatory action against paw edema. The results revealed that each drug’s effect was significant (*p<0.05) among each group. It can be demonstrated that of the six imidazole derivative compounds that were investigated, compounds C1IN, C2IN, and C4IN demonstrated a significant positive effect against carrageenan-induced inflammation in the hind paw. The effects of these two substances were more akin to those of the widely used drug indomethacin. When compared to the toxic group, each treated compound’s influence was found to be essential. Furthermore, research indicates that compounds C1IN and C2IN showed significant effect even from compound C4IN and C5IN. During examination, the compounds such as C10IN, and C11IN also showed significant action against the edema induced by carrageenan. Hence it can be demonstrated that the components may be effective components against different types of inflammatory cytokines that cause inflammation. The results of the investigation are shown in Figure 16.
 |
Figure 16: Imidazole derivatives’ anti-inflammatory properties against carrageenan-induced paw edema. |
Assessment of inflammatory cytokines
The assessment of cytokines is a crucial aspect of understanding the immune response and inflammatory processes within the body. TNF-α and IL-1β, play pivotal roles in mediating and regulating immune responses. The measurement of these cytokines in blood serum provides valuable insights into the extent and nature of inflammation.
Various methods, including ELISA, are commonly employed to quantify the levels of ILs in blood serum. ELISA kits designed for specific cytokines allow for accurate and sensitive measurements, enabling researchers to assess the immune system’s activity. Elevated levels of these cytokines often indicate an inflammatory response, which can be associated with various conditions such as infections, autoimmune diseases, and inflammatory disorders. The assessment of inflammatory cytokines in blood serum not only contributes to the diagnosis and monitoring of inflammatory conditions but also serves as a valuable tool in understanding the mechanisms underlying immune responses. It is rely on these assessments to tailor therapeutic interventions and develop targeted treatments for conditions characterized by dysregulated immune and inflammatory processes.
In this study, anti-inflammatory potential of components was examined via assessing TNF-α and IL-1β as anti-inflammatory cytokines. The plasma that was extracted from the treated rats’ blood was used determine inflammatory cytokines. According to the study’s findings, compounds C1IN, C2IN, C2IN, C5IN, and C11IN are the most notable imidazole Schiff base derivatives that are active against ILs and counteract carrageenan-induced toxicity. It was discovered that the test drug’s effects were similar to those of the standard medication. The study’s conclusions is depicted in Figure 17.
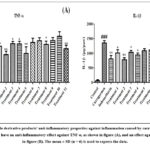 |
Figure 17: Imidazole derivative products’ anti-inflammatory properties against inflammation caused by carrageenan. |
Discussion
Carrageenan model is to the study acute inflammation in laboratory animals, particularly rodents. In this model, the animal’s paw’s subcutaneous tissue is injected with carrageenan, a polysaccharide derived from seaweed, to cause inflammation. A localized inflammatory response triggered by carrageenan is marked by the production of inflammatory mediators such as prostaglandins, histamines, and cytokines. The inflammation peaks a few hours after carrageenan administration and gradually subsides. Researchers assess the efficacy of potential anti-inflammatory agents by monitoring the reduction in paw edema, providing valuable insights into the substances’ anti-inflammatory effects. Carrageenan-induced paw edema serves as a reliable tool for screening and evaluating anti-inflammatory compounds and understanding the mechanisms involved in acute inflammation 24–26.
Schiff’s base imidazole derivatives have demonstrated notable anti-inflammatory effects in various experimental studies. These compounds, formed through the condensation of imidazole and aldehydes or ketones, exhibit pharmacological activities, including anti-inflammatory properties. The anti-inflammatory effect is often assessed using models like carrageenan-induced paw edema. In experimental setups, these derivatives have shown the ability to attenuate the inflammatory response, reducing factors such as paw swelling and cytokine levels 27–31. The precise mechanisms through which Schiff’s base imidazole derivatives exert their anti-inflammatory effects may involve modulation of inflammatory mediators, inhibition of pro-inflammatory cytokines, or interference with pathways implicated in inflammation. The anti-inflammatory potential of Schiff’s base imidazole derivatives is of interest in drug development, suggesting a role in mitigating inflammatory conditions 32,33. Further research is essential to elucidate the specific molecular mechanisms, signaling pathways, and potential therapeutic applications of these derivatives in the context of inflammation-related disorders. 34.
It is also reported that imidazole components with their Schiff base fragment exhibit significant effect against pathogenic fungi, including A. fumigatus, S. apiospermum and C. albicans. The azole components exhibited greatest antimicrobial activities against C. albicans. The examined ligands’ capacity to scavenge radicals composed of DPPH was also examined 32. In our outcome of the study it has been demonstrated that imidazole derivatives such as C1IM, C2IM, C4IM and C11IM exhibited potential anti-inflammatory activity against inflammation induced by carrageenan. Although compound C10IM exhibited comparatively similar action as exhibited by the compound C2IM and C4IM.
Sharma et al., 2021 established that imidazole molecules possess the capability to interact with a diverse array of targets, including G-quadraplexes, p53-Murine Double Minute 2 (MDM2) protein, tyrosine and serine-threonine kinases, histone deacetylases, and microtubules. The review delves into crucial aspects of structure-activity relationships and explores imidazole-containing compounds that demonstrate anticancer activity, often through mechanisms that are not fully elucidated. The intention of this review is to stimulate the conceptualization and synthesis of novel anticancer molecules, providing a comprehensive overview of recent advancements in the discovery and development of anticancer drugs based on imidazole 35
As reported in the study, newly synthesized derivatives of imidazole and metronidazole demonstrated notable antibacterial activity. The study focused on innovative modifications of imidazole rings, incorporating 1,2,4-triazole moieties, 1,3-oxazoline, thiadiazole, oxadiazole, and Schiff’s bases to create novel derivatives. The Compounds such as aminobiimidazol-4-one fused with hydrazine hydrate, after being transformed into a number of derivatives, compounds 7a-c and 10a-c were found to have strong antibacterial activity 36. Based on the modifications to the structure of apcin, a switched 2-aminopyrimidine compound, Huang et al. created new purine and pyrimidine molecules. Imidazole compound is a particular inhibitor of Cdc20, a protein that overexpresses in a number of cancers and activates the essential E3 ubiquitin ligase APC/C that regulates the progression of the cell cycle 37.
Pyridinyl imidazoles, and their metabolite effect on T-cell and B-cell mediated immune system reactions with corticosteroid dexamethasone and the fungal macrolide immunosuppressives rapamycin, and cyclosporin A exhibits anti-inflammatory capabilities using a delayed type hypersensitivity (DTH) response murine model that is specific to specific antigens. Because pyridinyl imidazoles can separately reduce the inflammatory components of a disease without causing systemic immunosuppression, they are therefore better candidates for the treatment of chronic inflammatory diseases, especially those which are caused by cytokines (e.g., TNF, IL-1), eicosanoids, and other known arbitrators of inflammation 29.
Conclusion
In conclusion, the current investigation establishes that certain Schiff’s base Imidazole derivatives, particularly compounds like C1IN, C2IN, C4IN, C5IN, and C11IN, exhibit noteworthy efficacy in mitigating paw edema and reducing the level of TNF-α and IL-1β. This outcome underscores the potential of the developed Schiff’s base Imidazole derivatives as viable alternatives for anti-inflammatory intervention. The observed reduction in paw edema and cytokine levels suggests a promising anti-inflammatory profile for these compounds, positioning them as candidates for further exploration and development. The study’s findings contribute valuable insights into the anti-inflammatory properties of Schiff’s base Imidazole derivatives, paving the way for potential therapeutic applications in inflammatory conditions. Future research endeavors should delve deeper into the mechanistic aspects and conduct additional preclinical studies to validate the translational potential of these derivatives in the realm of anti-inflammatory pharmacotherapy.
Acknowledgment
The authors thanks Amity Institute of Pharmacy, Noida, U.P, 201303 for facilitating the utilities in completion of the study.
Conflict of interest
No conflict of interest is declared by the authors.
Funding Sources
There is no funding sources
References
- Hoffman, S.B. Compend. Contin. Educ. Pract. Vet. 2001, 23:464–473.
- Alshibl, H.M.; Al-Abdullah, E.S.; Haiba, M.E.; Alkahtani, H.M.; Awad, G.E.A.; Mahmoud, A.H.; Ibrahim, B.M.M.; Bari, A.; Villinger, A. Molecules 2020, 25(14):3251.
CrossRef - Zhang, L.; Bao, M.; Liu, B.; Zhao, H.; Zhang, Y.; Ji, X.Y.; Zhao, N.; Zhang, C.; He, X.; Yi, J.; et al. Pharmacology 2020. 105(3-4):123-34.
CrossRef - Gaurav. Pharmacogn. Mag. 2022, 18, 548–558,
- DeLong, E.F.; Pace, N.R. Syst. Biol. 2001, 50(4):470-478.
CrossRef - Rademacher, F.; Dreyer, S.; Kopfnagel, V.; Gläser, R.; Werfel, T.; Harder, J. The Front. Immunol. 2019.10.
CrossRef - Lian, W.S.; Wang, F.S.; Chen, Y.S.; Tsai, M.H.; Chao, H.R.; Jahr, H.; Wu, R.W.; Ko, J.Y. Biomedicines 2022. 10(4):860.
CrossRef - Abdulrahman LK, Al-Mously MM, Al-Mosuli ML, Al-Azzawii KK. Int. J. Pharm. Pharm. Sci. 2013. 5:494-504.
- Lee, C.; Wilderman, R.; Park, J.W.; Murphy, T.J.; Morgan, E.T. Mol. Pharmacol. 2020, 98(3):267-79.
- Dhingra, A.K.; Chopra, B.; Jain, A.; Chaudhary, J. Mini-Reviews Med. Chem. 2022, 22(10):1352-73.
CrossRef - Slassi, S.; Aarjane, M.; Amine, A. J. Mol. Struct. 2022, 5;1255:132457.
CrossRef - Abdulghani, S.M.; Al-Rawi, M.S.; Tomma, J.H. Chem. Methodol. 2022.
- Sameer Al-Rawi, M.; Mohammed Abdulghani, S.; Hermiz Tomma, J. Chem. Methodol. 2022.
- Vidhya, K.; Kumar, K.P.; Piramanayagam, S.; Arulkumar, M.; Balraj, J.; Jairaman, K.; Subashini, G.; Angayarkanni, J. J. Mol. Struct. 2022: 1-10.
- Suliman, R.S.; Alghamdi, S.S.; Ali, R.; Rahman, I.; Alqahtani, T.; Farah, I.; Aljatli, D.A.; Hwaizi, S.; Algheribe, S.; Alehaideb, Z.; et al. Molecules 2022,
- Da Silva Guerra, A.S.H.; Do Nascimento Malta, D.J.; Morais Laranjeira, L.P.; Souza Maia, M.B.; Cavalcanti Colaço, N.; Do Carmo Alves De Lima, M.; Galdino, S.L.; Da Rocha Pitta, I.; Gonçalves-Silva, T. Int. Immunopharmacol. 2011, doi:10.1016/j.intimp.2011.07.010.
CrossRef - Haghighijoo, Z.; Firuzi, O.; Meili, S.; Edraki, N.; Khoshneviszadeh, M.; Miri, R. Iran. J. Pharm. Res. 2020, doi:10.22037/ijpr.2019.1100909.
- Ceramella, J.; Iacopetta, D.; Catalano, A.; Cirillo, F.; Lappano, R.; Sinicropi, M.S. Antibiotics 2022.
- Yadav, S.; Kumar, N. Orient. J. Chem. 2021, doi:10.13005/ojc/370517.
CrossRef - Kumar, V.; Ain, S.; Kumar, B.; Ain, Q. Int. J. Pharm. Res. 2020, doi:10.31838/ijpr/2020.SP2.169.
CrossRef - Jargalsaikhan, B.E.; Ganbaatar, N.; Urtnasan, M.; Uranbileg, N.; Begzsuren, D. Biomed. Pharmacol. J. 2019, 12, 1801–1809, doi:10.13005/bpj/1811.
CrossRef - Amdekar, S.; Roy, P.; Singh, V.; Kumar, A.; Singh, R.; Sharma, P. Int. J. Inflam. 2012, doi:10.1155/2012/752015.
CrossRef - Umar, S.; Zargan, J.; Umar, K.; Ahmad, S.; Kant, C.; Khan, H.A. Chem. Biol. Interact. 2012, 197, 40–46, doi:10.1016/j.cbi.2012.03.003.
CrossRef - Maswadeh, H.M.; Semreen, M.H.; Naddaf, A.R. Acta Pol. Pharm. – Drug Res. 2006.
- Chaudhari, S.P.; Baviskar, D.T. Adv. Tradit. Med. 2021, doi:10.1007/s13596-020-00502-1.
CrossRef - Odira, H.O.; Mitema, S.O.; Mapenay, I.M.; Moriasi, G.A. J. Evidence-Based Integr. Med. 2022, doi:10.1177/2515690X221082986.
CrossRef - Faisal, M.A.; Oktaviyanti, I.K.; Sujuti, H.; Rudijanto, A. Open Access Maced. J. Med. Sci. 2020, doi:10.3889/oamjms.2020.5505.
CrossRef - Sumarlin, L.O.; Ernita, N.; Afandi, F.R.; Fathoni, A. Sci. Technol. Indones. 2021, doi:10.26554/STI.2021.6.2.74-84.
CrossRef - Reddy, M.P.; Webb, E.F.; Cassatt, D.; Maley, D.; Lee, J.C.; Griswold, D.E.; Truneh, A. Int. J. Immunopharmacol. 1994, doi:10.1016/0192-0561(94)90053-1.
CrossRef - Umarani, N.; Ilango, K.; Garg, G.; Srinivas, B.K.; Hemalatha, V. Int. J. Pharm. Pharm. Sci. 2011.
- Singh, B.; Jana, S.K.; Ghosh, N.; Das, S.K.; Joshi, M.; Bhattacharyya, P.; Chaudhury, K. J. Pharm. Biomed. Anal. 2017;132:103-8.
CrossRef - Slassi, S.; Fix-Tailler, A.; Larcher, G.; Amine, A.; El-Ghayoury, A. Heteroat. Chem. 2019, doi:10.1155/2019/6862170.
CrossRef - Lashanizadegan, M.; Nikoofar, K.; Aghaei, A.; Mehrikaram, F.; Mirzazadeh, H. Solid State Sci. 2019,
- Chiu, Y.J.; Lin, C.H.; Lin, C.Y.; Yang, P.N.; Lo, Y.S.; Chen, Y.C.; Chen, C.M.; Wu, Y.R.; Yao, C.F.; Chang, K.H.; et al. Int. J. Mol. Sci. 2023, doi:10.3390/ijms24032642.
CrossRef - Sharma, P.; Larosa, C.; Antwi, J.; Govindarajan, R.; Werbovetz, K.A. Molecules 2021, doi:10.3390/molecules26144213.
CrossRef - Atia, A.J.K. Molecules 2009, doi:10.3390/molecules14072431.
CrossRef - Wang, L.; Zhang, J.; Wan, L.; Zhou, X.; Wang, Z.; Wei, W. Pharmacol. Ther. 2015.

This work is licensed under a Creative Commons Attribution 4.0 International License.










