Preparation and Characterization of AB2-Type Miktoarm Star-Shaped Nanomaterials Having Lactic Acid as an Arm
Department of Chemistry, School of Sciences, Gujarat University, Ahmedabad, Gujarat, India.
Corresponding Author E-mail: dvvasava@gujaratuniversity.ac.in
DOI : http://dx.doi.org/10.13005/ojc/400131
Article Received on : 23 Nov 2023
Article Accepted on : 19 Jan 2024
Article Published : 31 Jan 2024
Reviewed by: Dr. Abdelwahab Omri
Second Review by: Dr. Nenad Ignjatovic
Final Approval by: Dr .N. P. Subiramaniyam
A series of seven AB2-type hydrophobic miktoarm star polymeric nanomaterials were nanofabricated using coupling-onto approach. These tridentate nano-stars were synthesized using carbodiimide chemistry and characterized using FT-IR, 1H NMR and DLS techniques. Lactic acid is chosen as “arm A” and amino adipic acid as “core”, while seven biodegradable synthetic amides and polyamides as “arm B” were used individually. The diameter of the smallest nano-star LCE was 558.6 nm and largest nano-star LTD was 733.3 nm. The presence of long aliphatic chain in the LDE and LDD as well as presence of aromatic ring in LTE and LTD give them a bigger diameter in comparison to LCE, LLE and LLD. TGA analysis of the product reported a significant loss of 35% by total mass at 400ᵒC, indicating thermal degradation. The products proved to be biodegradable after 15 days of biological treatment. Biodegradation was structurally confirmed by FT-IR analysis of degraded samples.
KEYWORDS:AB2-type star; Biodegradability; Carbodiimide Chemistry; Coupling-onto
Download this article as:| Copy the following to cite this article: Panchal S. S, Vasava D. V. Preparation and Characterization of AB2-Type Miktoarm Star-Shaped Nanomaterials Having Lactic Acid as an Arm. Orient J Chem 2024;40(1). |
| Copy the following to cite this URL: Panchal S. S, Vasava D. V. Preparation and Characterization of AB2-Type Miktoarm Star-Shaped Nanomaterials Having Lactic Acid as an Arm. Orient J Chem 2024;40(1). Available from: https://bit.ly/4bfVJ4m |
Introduction
Biodegradable polymeric materials (BPMs) have been appealing research consideration from the last four decades due to ecological fouling caused by conventional polymers.1 Synthesizing BPMs, either chemically, biologically or modification is a necessity owing to the difficulty in obtaining reproducibility when using natural polymeric materials because our natural resources are finite.2
Hetero-arm star polymers, as name suggests, are structures having asymmetric polymeric chains combined from one end at a core to form star shape.3 They are also called miktoarm star polymers (MSPs). BPM-based MSPs are better candidates for passive targeting as they have smaller micelle sizes and lower critical micelle concentration (CMC) values,4 allowing them to disassemble at high dilution (e.g., in the bloodstream).5 The induced self-assembly capability facilitates the formation of nano- and microemulsions, thereby enhancing a wide range of theragnostic applications for MSPs.6 Considering the many applications of biodegradability induced MSPs, like gene or drug delivery,7 promising results can be achieved compared to traditional linear and branched polymers.8 This potency can be achieved by selecting BPMs as arms with desirable characteristics such as biocompatibility,9 hydrophobicity,10 zeta-potential,11 and various more while designing and synthesizing MSPs.12 Unlike branched polymers, synthesis of MSPs is more feasible as the dense shell structures are reduced to form linear arms that can possess a maximum arm length, thus reasonably minimizing the steric hindrance.13
Fabricating approaches for BPMs based MSPs mainly include: (1) core-first14 (2) arm-first15 (3) coupling-onto.16 In coupling-onto approach, a variety of polymeric arms are synthesized and then coupled with a multifunctional core. The number of arms in MSPs synthesized using coupling-onto approach depends on the active coupling sites of the multifunctional core.17
The aim of this work is to design, synthesize and characterize a series of seven tridentate AB2 type miktoarm star-shaped polymeric nanomaterials through coupling-onto approach using carbodiimide chemistry that has lactic acid as the A arm and biodegradable synthetic amides and polyamides as the B arm.
Experimental section
Materials
Lactic acid (2-hydroxypropionic acid) was purchased from Merck, USA and used as received. N-Hydroxysuccinimide (NHS) and 1-ethyl-3-(3-dimethylaminopropyl) carbodiimide hydrochloride (EDC) were purchased from Tokyo Chemical Industry Co., Ltd. (TCI), Japan. Aminoadipic acid (AAA), triethylamine (TEA), N, N-dimethylacetamide (DMAc) and N, N-diisopropylethylamine (DIEA) were purchased from Sisco Research Laboratories Pvt. Ltd. (SRL), Mumbai. Dichloromethane (DCM), dimethyl sulfoxide (DMSO), chloroform (CH3Cl), diethyl ether, methanol and acetone were purchased from Finar by Actylis, Gujarat, India. All solvents were distilled before use.
Synthesis Methods
Lactic acid (LA), an organic α-hydroxy acid that is readily miscible in water is selected as arm A. Seven biodegradable amides and polyamides synthesized via Yamazaki-Higashi phosphorylation were considered for arm B of the MSPs. Acrylic acid and lactic acid were amine terminated to form amides (AcA-EDA, LA-EDA, and LA-DAP) whereas adipic acid and terephthalic acid were amine terminated to form polyamides (AdA-EDA, AdA-DAP, TA-EDA, TA-DAP) having varied chain length.
Synthesis of NHS activated lactic acid (LA-NHS)
The carboxylic group of lactic acid (LA) was activated to form LA-NHS using carbodiimide chemistry (Fig 1). LA in anhydrous DCM (2 mL) was treated with a solution of excess NHS and EDC in anhydrous DCM at constant stirring for 24 h at room temperature in nitrogen atmosphere. The mole ratio of LA:NHS:EDC was calculated to be 1:5:5. The resulting mixture was precipitated with diethyl ether (2 mL) and centrifuged at 4000 rpm for 5 min. After discarding the supernatant, the product was dissolved in DCM and reprecipitation with diethyl ether was repeated. The precipitates were stored at -20°C after removal of the residual solvents under vacuum.
Synthesis of aminoadipic acid conjugated lactic acid (LA-AAA).
LA-NHS was coupled with the core amino adipic acid (AAA) to yield LA-AAA (Fig 1). A clear solution of AAA dissolved in DMSO (1 mL) by heating to 150°C was prepared. After the solution attained room temperature, LA-NHS and TEA were gradually added to it. The reaction was stirred at room temperature for 24 h in nitrogen atmosphere. The mole ratio of LA-NHS:AAA:TEA was calculated to be 1:1:1. The solution was then precipitated with distilled water (3 mL) and the precipitates were extracted with chloroform (2 mL). The chloroform extract was vacuum filtered and concentrated in a rotary evaporator. The resultant solution was precipitated with diethyl ether (2 mL) and centrifuged at 4000 rpm for 5 min. The precipitates were stored at room temperature after removal of the residual solvents under vacuum.
Synthesis of NHS activated LA-AAA (LA-AAA-(NHS)2)
The two carboxylic groups on AAA segment of LA-AAA were activated to form LA-AAA-(NHS)2 using carbodiimide chemistry as earlier (Fig.1). LA-AAA in anhydrous DCM (2 mL) was treated with a solution of NHS and EDC in anhydrous DCM at constant stirring for 24 h at room temperature in nitrogen atmosphere. The mole ratio of LA-AAA: NHS: EDC was calculated to be 1:10:10. The resulting mixture was precipitated with diethyl ether (2 mL) and centrifuged at 4000 rpm for 5 min. After discarding the supernatant, the product was dissolved in DCM and reprecipitation with diethyl ether was repeated. The precipitates were stored at -20°C after removal of the residual solvents under vacuum.
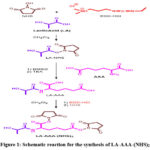 |
Figure 1: Schematic reaction for the synthesis of LA-AAA-(NHS)2. |
Synthesis of AB2-type miktoarm star polymer
The seven synthesized biodegradable amides and polyamides were reacted with the two activated sites of LA-AAA-(NHS)2 moiety by modifying a reported process to yield a series of seven novel AB2-type miktoarm star polymer (Fig.2). A solution of LA-AAA-(NHS)2 in anhydrous DCM (2 mL) was prepared. The amides and polyamides i.e.: AcA-EDA, LA-EDA, LA-DAP, AdA-EDA, AdA-DAP, TA-EDA, and TA-DAP were then added to the solution individually along with excess DIEA. The solution was left to stir for 48 h under a nitrogen atmosphere at room temperature. The resultant mixture was precipitated with a 2:1 v/v solution of diethyl ether: methanol and centrifuged at 8000 rpm for 10 min. After discarding the supernatant, the product was dissolved in DCM and reprecipitation with diethyl ether: methanol was repeated. The residual solvents were removed under vacuum and resultant product was stored at room temperature.
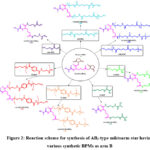 |
Figure 2: Reaction scheme for synthesis of AB2-type miktoarm star having various synthetic BPMs as arm B. |
Synthesis of AB2-type miktoarm star polymeric nanomaterial
The synthesized AB2-type MSPs were treated with ultrasonication for their nanoscale attainment. Suspension solutions (0.5mg/mL) of each MSPs was prepared in three different solvents: diethyl ether, chloroform and DMSO individually. The solutions were emulsified in ultrasonic bath on 400 watts agitation at 20°C temperature for 5 min. The resultant nano-emulsions were stored at -40°C to stabilize.
Characterization
The structural confirmations of synthesized MSPs were conducted using Fourier-transform infrared spectroscopy (FT-IR) performed on Perkin Elmer Spectrum 2 spectrophotometer from 400 to 4000 cm-1 and proton nuclear magnetic resonance (1H NMR) spectra recorded on Bruker AVANCE III 500 MHz using CDCl3 as solvent and TMS as internal standard. The particle size, size distribution and polydispersity index (PDI) of the nanomaterials were measured by a dynamic light scattering (DSC) on Zetasizer Nano ZSP (ZEN 5600) instrument. Approximately 1 mg of sample was dispersed in 1.5 mL of ethanol and transferred into a 40 μL micro cuvette. The degradation of the synthesized MSPs was conducted both thermally and biologically. Thermal degradation was performed on HiRes1000 Thermal Gravimetric Analyzer (TGA) having temperature range from room temperature to 1100ᵒC with heating rate of 0.01 to 200°C/min. The MSPs were biodegraded using selected species of microorganisms, having known cultured microbes, soil microbes, chitinase enzymes and extracellular enzymes individually. This biological degradation was confirmed structurally via FT-IR.
Results and discussion
Structural confirmations
FT-IR
Fig.3 represents the characteristic FT-IR spectra of all the synthesized MSPs. The noticeable absorption band at approximately 2996 cm-1 is the asymmetric stretching vibrations of -CH3 and 3265 cm-1 is for -O-H stretching in LA. An absorption band at 1737 cm-1 was due to carbonyl -C=O stretching vibration, whereas stretching vibrations at approximately 3335 cm-1 and bending vibrations at around 1587 cm-1 were observed due to amine -N-H segments for amide groups.
 |
Figure 3: FT-IR spectra of synthesized MSPs. |
1H NMR
Fig. 4 shows 1H-NMR spectrum having the peaks corresponding to confirm the structures of the synthesized MSPs given besides. The signals that occur at 7.4 (t) ppm are associated with amide O=C-N-H group and signals at approximate 1.24 (S) ppm are associated with C-C-H segment in LA (arm A) in all the three MSPs. In LLE, the signal appearing in a region at approximately 7.01 (d) ppm results from the -O-H protons, whereas signal at 2.09 (s) ppm results from the proton of carbonyl O=C-C-H segment of arm B, LA-EDA. In LDE, the signal at 6.43 (s) ppm represents protons of terminal amines C-N-H segment, whereas the signal at 1.91 (q) ppm represents protons of alkanes C-C-H segment from arm B, AdA-EDA. In LTD, the signals between 6.5 to 8.5 ppm shows signal for aromatic proton as well as the signal in region at 3.18 (s) ppm shows protons of terminal amines C-N-H segment, whereas the signal at 2.29 (t) ppm represents protons of alkanes C-C-H segment from arm B, TA-DAP. The very sharp intensive peak of solvents are at 3.35 ppm due to exchange of protons with deuterium water (D2O) and 2.50 to 2.52 ppm represents deuterated DMSO.
 |
Figure 4: 1H NMR spectra of AB2 stars having lactic acid as arm A |
Solubility
The solubility of these hydrophobic MSPs was determined by the dissolution of 0.2 mg of sample in 1 mL of solvent (10 wt%) at room temperature, as summarized in Table 1. The results show that all the MSPs are soluble only in DCM, whereas partially soluble in DMSO.
Table 1: Solubility of diamides and polyamides
|
Solvents (Decreasing polarity) |
CCE |
CLE |
CDE |
CTE |
CLD |
CDD |
CTD |
|
DMSO |
++- |
++- |
++- |
++- |
++- |
++- |
++- |
|
NMP |
— |
— |
— |
— |
— |
— |
— |
|
MeOH |
— |
— |
— |
— |
— |
— |
— |
|
CHCl3 |
— |
— |
— |
— |
— |
— |
— |
|
THF |
— |
— |
— |
— |
— |
— |
— |
|
DCM |
+++ |
+++ |
+++ |
+++ |
+++ |
+++ |
+++ |
|
Diethyl ether |
— |
— |
— |
— |
— |
— |
— |
+++: highly soluble, ++-: partially soluble, +–: scarcely soluble, —: insoluble
Particle-size
The particle-size of all the nano stars were measured using DLS method. Fig 5 shows size distribution graph by intensity. The dimensions of all seven nano stars LCE, LLE, LLD, LDE, LDD, LTE and LTD are 558.6, 593.7, 616.2, 630.4, 679.9, 723.5 and 733.3 respectively. In principle, materials having 1-1000 nm size in at least one dimension term as nanomaterials.18 The diameters od all the products are less than 999 nm, hence confirming their nano dimensions for polymers. The particle-size of the products shows comparative elevation in the products (LTD, LDD and LTD) having DAP segments with respect to the products (LCE, LLE, LDE and LTE) of EDA segment, due to the presence of one more carbon in their diamine aliphatic chain. The particle-size of the products increases substantially according to the increase in the chemical structure of the “arm B” respectively. The presence of aliphatic chain in the arms of LDE and LDD and aromatic ring in the arms of LTE and LTD corresponds to comparatively bigger particle-size of products.
Also, the Z-average size and PDI of the products are also shown in table 2. The PDI of the products ranges from 0.282 to 0.714.
 |
Figure 5: DLS results of all MSPs |
Degradation
Thermal degradation
Fig.6 shows the thermally degrading behavior of synthesized MSP during the TGA. Small molecules like water, carbon dioxide and ammonia were released before 100ᵒC. A substantial decrease in percentage mass of all MSPs can be witnessed near 200ᵒC. The percentage mass of products lost around 400ᵒC is about 30%. As the temperature increases above 400ᵒC gradual percentage mass loss can be seen before the product turns into char after 700ᵒC. The three MSPs LCE, LLE and LLD show a mild curve indicating gradual mass loss due to terminated arm B, whereas LDE, LDD, LTE and LTD show sudden drop in mass loss at 400ᵒC. Also, the char yield of LTE and LDD starts forming earlier with respect to other due to the presence of aromatic carbons.
 |
Figure 6: Thermogram of MSPs |
Biological degradation
10 mg of synthesized products was incubated, with selected microorganisms for up to 15 days in shaking condition at 37ᵒC. The samples were kept controlled by not adding any microorganisms; and kept isolated ingrowth media. The samples were analyzed visually under microscope and structurally via FT-IR at an interval of 5 days. In fig.7, all the samples show visual degradation at the end of 15 days biodegradation treatment procedure.
 |
Figure 7: Samples at day 15th of biological degradation treatment. |
Fig.8 shows the FT-IR graph of the biodegraded MSPs taken after completion 15 days. The graphs significantly show the diminishing of all peaks denoting amides, amines as well as carboxylic acids, which conclude that the samples have been degraded successfully.
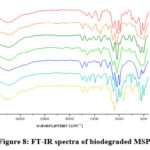 |
Figure 8: FT-IR spectra of biodegraded MSPs. |
Conclusion
Coupling-onto approach of nanofabrication was used to synthesis a series of seven tri-dentate AB2-type miktoarm star polymeric nanomaterials by carbodiimide chemistry. The synthesized MSPs consists of lactic acid as “arm A” and synthetic biodegradable amides and polyamides containing acrylic acid, lactic acid, adipic acid and terephthalic acid fragments, as “arm B” having aminoadipic acid as “core”. These synthesized nano star polymers were confirmed by FT-IR, 1H NMR and DLS. The thermal degradation of these products was studied using TGA. In addition, samples were biologically treated for biodegradation. The structural degradation was confirmed by FT-IR.
The hydrophobic MSPs range in diameter from 558.6 nm to 733.3 nm. LCE has the smallest particle-size with a diameter of 558.6 nm and LTD has the largest particle-size with diameter of 733.3 nm. Furthermore, PDI <1.0 indicates that all MSPs are almost monodisperse. About 30% of the total mass goes near 400ᵒC, which defines the thermal decomposition of MSPs. The FT-IR graphs after biological degradation confirm that the products have biodegraded successfully within 15 days by showing a significant diminish in the polyamide peaks when compared to FT-IR graphs before biological degradation.
Acknowledgment
The authors acknowledge Department of Chemistry, School of Sciences, Gujarat University, Ahmedabad, Gujarat, India for basic infrastructural facilities. The authors also acknowledge the characterization conveniences of Materials Research Centre, MNIT Jaipur for IR and DLS; Institute Instrumentation Centre, IIT Roorkee for NMR; University Science Instrument Centre, Delhi University for TGA and Department of Biochemistry and Forensic Science, School of Sciences, Gujarat University for biodegradation.
Conflicts of interest
There are no conflicts to declare.
References
- Nanok, T., Khanom, N., Hormnirun, P., Chansaenroch, C., and Laobuthee, A. ChemistrySelect. 2023, 8, e202301046
CrossRef - Panchal, S. S., and Vasava, D. V. ACS Omega. 2020, 5, 4370-4379
CrossRef - Levi, A. E., Fu, L., Lequieu, J., Horne, J. D., Blankenship, J., Mukherjee, S., Zhang, T., Fredrickson, G. H., Gutekunst, W. R., and Bates, C. M. Macromolecules. 2020, 53, 702-710
CrossRef - Park, J., Ahn, N. Y., and Seo, M. Polymer Chemistry. 2020, 11, 4335-4343
CrossRef - Shao, L., Liu, N., Wang, Z., Zhan, P., Zhang, L., and Wu, Z. ChemistrySelect. 2023, 8, e202301633
CrossRef - Kupczak, M., Mielanczyk, A., and Neugebauer, D. Materials (Basel). 2021, 14, 1-14
CrossRef - Panchal, S. S., and Vasava, D. V. International Journal of Polymeric Materials and Polymeric Biomaterials. 2023, 1-12
- Yang, Y.-L., Tsao, H.-K., and Sheng, Y.-J. Macromolecules. 2020, 53, 594-601
CrossRef - Hajebi, S., Yousefiasl, S., Rahimmanesh, I., Dahim, A., Ahmadi, S., Kadumudi, F. B., Rahgozar, N., Amani, S., Kumar, A., Kamrani, E., Rabiee, M., Borzacchiello, A., Wang, X., Rabiee, N., Dolatshahi-Pirouz, A., and Makvandi, P. Adv Healthc Mater. 2022, 11, e2201583
CrossRef - Ikkene, D., Arteni, A. A., Ouldali, M., Francius, G., Brulet, A., Six, J. L., and Ferji, K. Biomacromolecules. 2021, 22, 3128-3137
CrossRef - Silva, C., Di-Medeiros, M. C. B., Liao, L. M., Fernandes, K. F., and Batista, K. A. Materials (Basel). 2021, 14, 1-17
CrossRef - Adjuik, T. A., Nokes, S. E., and Montross, M. D. Journal of Applied Polymer Science. 2023, 140, 1-17
CrossRef - Wang, R., Damanik, F., Kuhnt, T., Jaminon, A., Hafeez, S., Liu, H., Ippel, H., Dijkstra, P. J., Bouvy, N., Schurgers, L., Ten Cate, A. T., Dias, A., Moroni, L., and Baker, M. B. Adv Healthc Mater. 2023, 12, 1-13
CrossRef - Panchal, S. S., and Vasava, D. V. International Journal of Polymeric Materials and Polymeric Biomaterials. 2021, 71, 1407-1424
CrossRef - Schwiertz, D., Holm, R., and Barz, M. Polymer Journal. 2019, 52, 119-132
CrossRef - Chafran, L., Matias, A. E., and Silva, L. P. ChemistrySelect. 2022, 7, e202201276
CrossRef - Chong, Y. K., Zainol, I., Ng, C. H., and Ooi, I. H. Journal of Polymer Research. 2019, 26, 1-15
CrossRef - Jeevanandam, J., Barhoum, A., Chan, Y. S., Dufresne, A., and Danquah, M. K. Beilstein J Nanotechnol. 2018, 9, 1050-1074
CrossRef

This work is licensed under a Creative Commons Attribution 4.0 International License.










