Potentially Biodynamic Tin(II) Complexes Derived from Macrocyclic Ligands: Synthesis, Spectral Analysis, In vitro Antibacterial, Antifungal and Brine Shrimp Lethality Evaluation
Anita Phor1 , Mamta2
, Mamta2 , Subhash2
, Subhash2 , J. S. Phor3
, J. S. Phor3 and Ashu Chaudhary2*
and Ashu Chaudhary2*
1Department of Chemistry, Hindu College Sonepat, Haryana, India.
2Department of Chemistry, Kurukshetra University, Kurukshetra, Haryana, India.
3Department of Physics, CRA College, Sonepat, Haryana, India.
Corresponding Author E-mail: ashuchaudhary21@gmail.com
DOI : http://dx.doi.org/10.13005/ojc/400110
Article Received on : 02 Oct 2023
Article Accepted on :
Article Published : 23 Feb 2024
Reviewed by: Dr. Prasanna Ranade
Second Review by: Dr. R. Gandhimathi
Final Approval by: Dr. Nenad Ignjatovic
Over the past several years, the predominance of biologically active macrocycles in medicinal chemistry has been increasing. Novel macrocyclic complexes of Sn(II) were synthesized by using macrocyclic ligands derived from various diamine and dicarboxylic acids in the ratio of 2:2. The structures of the obtained complexes were identified by various spectrometric methods such as Fourier transformation infra-red (FT-IR), nuclear magnetic resonance (NMR), electron spray ionization mass spectrometry, and powder X-ray diffraction. The spectral data provided evidence that the macrocyclic ligands acted as tetradentate ligands, forming coordination bonds with Sn(II) ions through the nitrogen atom of the imine (>C=N) group. The coordination complexes exhibited an octahedral geometry around the tin ion, with two chloro groups covalently attached. All of the synthesized compounds were evaluated for their biochemical properties, including antibacterial, antifungal, and Brine Shrimp Lethality. The antimicrobial potential of the macrocyclic compounds was examined against a panel of pathogenic microbes, including bacterial strains (Escherichia coli, Xanthomonas campestris, and Staphylococcus aureus) and fungal strains (Fusarium oxysporum and Aspergillus niger). The result showed that the macrocyclic complexes have remarkable antimicrobial potential as compared to their corresponding macrocyclic ligands. The positive findings of Brine shrimp lethality of the ligands and their complexes have been discussed.
KEYWORDS:Antibacterial; Antifungal; Brine shrimp lethality; Macrocyclic; Tin(II)
Download this article as:| Copy the following to cite this article: Phor A, Mamta M, Subhash S, Phor J. S, Chaudhary A. Potentially Biodynamic Tin(II) Complexes Derived from Macrocyclic Ligands: Synthesis, Spectral Analysis, In vitro Antibacterial, Antifungal and Brine Shrimp Lethality Evaluation. Orient J Chem 2024;40(1). |
| Copy the following to cite this URL: Phor A, Mamta M, Subhash S, Phor J. S, Chaudhary A. Potentially Biodynamic Tin(II) Complexes Derived from Macrocyclic Ligands: Synthesis, Spectral Analysis, In vitro Antibacterial, Antifungal and Brine Shrimp Lethality Evaluation. Orient J Chem 2024;40(1). Available from: https://bit.ly/3Ta2vSn |
Introduction
The rapid and extensive advancements in the field of coordination compounds, influenced by the unique scientific backgrounds, individual interests, and personal preferences of researchers, have been made accessible because of their widespread relevance in various current areas of interest, particularly in agriculture and medicine. The emergence of microbe’s resistance to the marketed drug is a global concern1,2. Microbes can easily alter their structure by mutating their genetic material and acquiring resistance to the already reported drugs. So, there is a requirement for new drugs with better efficacy to control the horrific devastation caused by pathogens. Combating against the virulence of pathogens, metal complexes have gained appreciable consideration over the years because of their relevancy with bioinorganic moieties3,4. Among all kinds of metal complexes, organotin(II) complexes concede substantial place based on their potential biological activities such as antimicrobial5, antidiabetic6, antioxidant7, antiinflammatory8, antitumor9, antimalarial10, antiproliferation11 and antituberculosis12. Organotin complexes are widely used in other areas also, such as they have been used in wood preservatives, antifouling paints, and surface disinfectants13.
The biochemical action of organotin(II) complexes were influenced by coordination number of metal atom, geometry, oxidation states, thermodynamic and kinetic stability of complexes. It is well known that tin metal form stable bond with the carbon as well as with heteroatoms and also exits in different coordination number from four to eight 14,15. The design of macrocyclic ligands and organotin complexes represents a relevant and qualified objective in the development of coordination chemistry. Due to synthetic flexibility, ease of synthesis and very good binding ability of amide group, macrocyclic ligands play an important role in inorganic chemistry to form stable complexes with most of the metal ions 16,17. Organotin compounds have been exploited widely in drug design and development due to their continuous increase in the field of pharmaceuticals, industrials, biological, analytical and medicinal areas 18-22.
Based on previous research on the coordinating properties of tetrazamacrocycles23-26, this work provides a concise description of new dimensions in the structural and biological activity, highlighting the efficiency of tetraaza macrocyclic ligands and their tin(II) complexes.
Experimental Section
Chemicals
All solvents and chemicals employed in this work were of analytical grade and used without further purification. “Methanol, malonic acid and glutaric acid, 1,8-Napthyridine-2,7-diamine, SnCl2 were procured from Merck.
Analytical Methods and Physical Measurements
The Rast Camphor method was utilized to determine the molecular weights. Conductivity measurements were conducted in dry dimethylformamide using a type 305 conductivity bridge. IR spectra were recorded on a Nicolet Magna FT-IR 550 spectrophotometer, employing KBr pellets. For 1H NMR spectra, a JEOL FX-90Q spectrometer was used with TMS serving as the internal standard. Tin was estimated gravimetrically as tin oxide. Powder X-ray diffraction data were collected on a Bruker AXS D8 Advance at diffraction angles 2θ = 10-90°. Additionally, carbon, hydrogen and nitrogen analyses were performed at the Central Drugs Research Institute (CDRI) in Lucknow.
Synthesis of the ligands (ML1-ML2)
In a 100 mL round bottom flask with a short neck, a measured quantity of 1,8-Napthyridine-2,7-diamine (1 mmol, 0.160 g) was placed. To this, an equivalent amount of malonic acid (1 mmol, 0.104 g) dissolved in methanol was supplementary. The reaction was carried out in equimolar ratios and animated underneath reflux for 11 hours. After completion, the reaction mixture was cooled, and the resulting off-white compound was purified through recrystallization using MeOH. The same procedure was employed for the synthesis of ML2, but with a different reagent. Instead of malonic acid, glutaric acid (1 mmol, 0.132 g) was used in the reaction.
Synthetic process of the complexes [Sn(ML1 )Cl2-Sn(ML2 )Cl2]
A mixture of ML1 (1 mmol, 0.456 g) and SnCl2 (1 mmol, 0.189 g) in a 1:1 ratio was prepared in MeOH and subjected to reflux heating for 42 hours. After cooling and left undisturbed for some time, resulting in the formation of a coloured complex that unglued out. The product was then washed and dried under P2O4, followed by recrystallization from a mixture of toluene and n-hexane in a 1:1 ratio. The same procedure was applied to synthesize [Sn(ML2)Cl2] using ML2 (1 mmol, 0.484 g) as ligands.
Antimicrobial Activity
Antibacterial Activity
The antibacterial activity of the newly synthesized macrocyclic compounds was assessed by the paper disc method against Escherichia coli, Xanthomonas campestris, and Staphylococcus aureus bacterial strains. For this technique, the petri-plates were used to pour the agar medium and left to solidify. Once solidified, the plates were stored upside down in the freezer to encourage water condensation on the higher closure. Synthesized complexes and ligands were dissolved in MeOH to create solutions at concentrations of 500 and 1000 ppm. To evaluate bacterial activity, two methods were used: In the first method, the paper discs were dipped in the test sample solution, and then placed on the seeded agar plates. In the second method, the paper discs were first placed on the seeded plates, and then the required amount of the test sample was pipetted onto the disc. The petri plates with the paper discs on the seeded agar were initially kept at a low temperature for two hours to allow for chemical diffusion. After this, the plates were incubated at the suitable optimum temperature (28 ± 2 °C) for 24-30 hours. Following the incubation period, the clear zone of inhibition around the treated disc was measured in millimeters. The standard antibiotic, Gentamicin, was used to compare the antibacterial screening results of the compounds.
Antifungal activity
The antifungal activity of the macrocyclic compounds was assessed using the spore germination technique against two pathogenic fungi, Fusarium oxysporum, and Aspergillus niger. Test compound solutions (0.5 mL) were prepared in DMF and applied on the fungus slides. The slides were then incubated for 24-72 hours at 37 °C. The diameter of the growth of fungi was measured after incubation period to determine the fungus’ linear growth, and the percentage inhibition was calculated as 100 x (C- T)/C, where C is the diameter of the fungal growth in the control plate after 96 h and T is the diameter of the fungal growth in the test plates after 96 h. The standard antibiotic, Bavistin, was used to compare the antifungal screening results of the compounds.
Brine Shrimp Lethality
For Brine Shrimp lethality27, synthesized ligands and complexes have been dissolved in dimethyl sulfoxide and pragmatic at different conc.: 5.0, 10.0, 20.0, 40.0, and 80.0 µg/ml. However, no more than 50 µL of dimethyl sulfoxide have been added vials containing the nauplii. A control group was also included for each concentration, consisting of a vial with the same amount of dimethyl sulfoxide and brine water, but without the test compound. The vials were examined under a microscope after 24 hours incubation period, and the quantity of living nauplii in each vials have been noted. The mean %age of nauplii mortality for each concentration was computed using these data.
Results and Discussion
All of the complexes were found to be monomeric, as evident from the determination of their molecular weights. The molar conductance values of the tin(II) complexes, measured in 10-3 M solutions, fall within the range of 11-25 ohm-1cm2mol-1, indicating their non-electrolyte nature. The physio-analytical data of tin(II) complexes are provided in Table 1.
Table 1: Physio-analytical data of tin(II) complexes.
|
Compounds |
M.P. (oC) |
Analysis, Found (Calcd.) % |
Mol. Wt. Found (Calcd.) |
||||
|
C |
H |
N |
Cl |
Sn |
|||
|
ML1 |
154-156 |
57.85 (57.89) |
3.50 (3.53) |
24.50 (24.55) |
– |
– |
456.35 (456.42) |
|
ML2 |
165-167 |
59.45 (59.50) |
4.12 (4.16) |
23.10 (23.13) |
– |
– |
484.45 (484.48) |
|
[Sn(ML1)Cl2] |
185-187 |
40.01 (40.09) |
2.44 (2.49) |
17.30 (17.34) |
10.91 (10.97) |
18.36 (18.37) |
646.00 (646.02) |
|
[Sn(ML2)Cl2] |
198-200 |
42.72 (42.76) |
2.94 (2.99) |
16.60 (16.62) |
10.49 (10.51) |
17.59 (17.61) |
674.02 (674.08) |
IR Spectra
The presence of amide groups at specific wavenumbers (1641–1671, 1431–1465, 1235–1250, and 570–660 cm-1) in the complexes strongly indicates the formation of closed cyclic products28. Furthermore, strong and sharp absorption bands observed in the regions of 2832–2860 and 1412–1438 cm-1 accredited to (C-H) stretching (ν) and bending (δ) vibrations, respectively29. The appearance of bands 350 & 370 cm-1 and 420 & 440 cm-1, corresponding to metal-chloride vibrations30. Table 2 provides a summary of the characteristic frequencies of the synthesized Sn(II) complexes.
Table 2: FT-IR Spectra information (cm-1) for the Tin(II) Complexes and the ligands.
|
Compounds |
Amide |
n(NH) |
n(Sn-Cl) |
n(Sn-N) |
|||
|
I |
II |
III |
IV |
||||
|
ML1 |
1641 |
1431 |
1232 |
570 |
3215 |
– |
– |
|
ML2 |
1680 |
1442 |
1265 |
560 |
3250 |
– |
– |
|
[Sn(ML1)Cl2] |
1650 |
1450 |
1240 |
550 |
3245 |
350 |
420 |
|
[Sn(ML2)Cl2] |
1671 |
1465 |
1260 |
660 |
3240 |
370 |
440 |
1H NMR Spectra
The tin(II) complexes were subjected to 1H NMR spectroscopy in DMSO-d6, and the conforming chemical shift values (δ) for diverse protons are recorded in Table 3. Several points reinforce the recommended geometry for these tin(II) complexes. The absence of any signals in the 1H NMR spectra of the complexes corresponding to amino and hydroxyl groups support the proposed structures. A broad signal detected in all complexes at δ 7.92 – 8.20 ppm can be attributed to the amide protons31. The aromatic ring protons of 1,8-Napthyridine-2,7-diamine are represented by the multiplets that appear in the range of 6.63–8.14 ppm. Malonic and glutaric acid’s methylene protons are attributed to singlets that show at ppm values of 2.86 – 2.90 and 3.20 – 3.23, respectively. The chemical shift values of the synthesized tin(II) compounds are presented in Table 3.
Table 3: 1H NMR (d, ppm) Spectral Data of the Ligands and their Tin(II) Complexes.
|
Compound |
(CO-NH) |
CO-CH2-CO |
CO-(CH2)3-CO |
|
ML1 |
7.90 |
1.95 |
– |
|
ML2 |
8.15 |
– |
3.20 |
|
[Sn(ML1)Cl2] |
8.20 |
2.06 |
– |
|
[Sn(ML2)Cl2] |
8.25 |
– |
3.40 |
Mass Spectra
The mass spectrum of the macrocyclic ligand [ML1] is represented in Fig. 1. The molecular formula of the macrocyclic ligand [ML2] is C22H18N8O4 (m/z = 456.35). The mass spectrum of macrocyclic ligand [ML1] displays molecular ion peaks corresponding to molecular ions [M]+ at m/z = 456.25. The empirical formula, as revealed by the elemental analysis, and the molecular ion peak [M]+ were in good agreement. The other fragmentation peak observed in the mass spectrum of macrocyclic ligand [ML1] was at 430.12 [C21H18N8O3]+, 416.01 [C20H16N8O3]+, 388.40 [C19H16N8O2]+, and 188.07 [C9H8N4O]+. The mass spectrum of the macrocyclic ligand [ML2] is represented in Fig. 2. The molecular formula of the macrocyclic ligand [ML2] is C24H20N8O4 (m/z = 484.45). The mass spectrum of macrocyclic ligand [ML2] displays molecular ion peaks corresponding to molecular ions [M]+ at m/z = 484.30. The empirical formula, as revealed by the elemental analysis, and the molecular ion peak [M]+ were in good agreement. The other fragmentation peak observed in the mass spectrum of macrocyclic ligand [ML2] was at 458.09 [C22H18N8O4]+, 444.60 [C21H18N8O4]+, 402.59 [C20H18N8O4]+, and 188.19 [C9H8N4O2]+.
 |
Figure 1: Mass spectrum of macrocyclic ligand [ML1]. |
 |
Figure 2: Mass spectrum of macrocyclic ligand [ML2]. |
Powder X-ray Diffraction Studies
The complex [Sn(ML2)Cl2] underwent an X-ray powder diffraction analysis to study its lattice dynamics. The recorded inter-planar spacing values (d, Å) and their corresponding Miller-0indices (h, k, l) (Table 4). The data indicate that the metal derivative possesses an orthorhombic lattice structure, with cell dimensions as follows: a = 17.8630, b = 7.4460, c = 15.7480, and α = β = γ = 90°.
Table 4: Powder X-ray diffraction data of [Sn(ML2)Cl2].
|
Peak No. |
2q (deg.) (obs.) |
d-spacing (obs.) Å |
h |
k |
l |
|
1 |
10.124 |
8.730 |
0 |
2 |
0 |
|
2 |
12.340 |
7.167 |
1 |
1 |
0 |
|
3 |
15.128 |
5.852 |
1 |
2 |
0 |
|
4 |
16.227 |
5.458 |
0 |
1 |
1 |
|
5 |
18.984 |
4.671 |
1 |
3 |
0 |
|
6 |
19.784 |
4.484 |
1 |
1 |
1 |
|
7 |
21.653 |
4.101 |
1 |
2 |
1 |
|
8 |
21.722 |
4.088 |
0 |
3 |
1 |
|
9 |
22.584 |
3.934 |
2 |
0 |
0 |
|
10 |
23.175 |
3.835 |
2 |
1 |
0 |
|
11 |
23.304 |
3.814 |
1 |
4 |
0 |
|
12 |
24.524 |
3.627 |
1 |
3 |
1 |
|
13 |
24.844 |
3.581 |
2 |
2 |
0 |
|
14 |
27.361 |
3.257 |
2 |
3 |
0 |
|
15 |
27.473 |
3.244 |
2 |
0 |
1 |
|
16 |
27.956 |
3.189 |
2 |
1 |
1 |
119Sn NMR SPECTRA
The inferences derived from IR and Proton NMR investigations are corroborated by the 119Sn NMR spectra. The complexes [Sn(ML1)Cl2] and [Sn(ML2)Cl2] exhibit signals at δ 561 and 542 ppm, respectively, indicating an octahedral environment around the tin atom in these complexes32. Based on the spectral evidence (Figure 3), the proposed structure for the [Sn(ML2)Cl2] complex can be suggested.
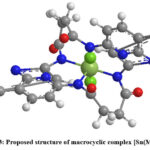 |
Figure 3: Proposed structure of macrocyclic complex [Sn(ML2)Cl2]. |
ANTI Microbial activities
Bacterial activity assessment was conducted using the paper disc method. The bacteria used in these experiments were Escherichia coli, Xanthomonas campestris, and Staphylococcus aureus. The zone of inhibition values for antibacterial activity is given in Table 5. The findings were evaluated in comparison with standard drugs, gentamicin for bacteria. The studied macrocyclic compounds appeared to be effective against all the tested bacterial strains. The macrocyclic complex [Sn(ML2)Cl2] showed higher antibacterial activity with zones of inhibition of 24.5 mm against S. aureus, 26.1 mm against E. coli, and 27.1 mm against X. campestris at 1000 ppm (Fig. 4).
The ligands and their corresponding metal complexes were assessed for their potential to inhibit the growth of selected fungi, Fusarium oxysporum, and Aspergillus niger, using the spore germination technique. The results of the antifungal activity, as indicated by the percentage of inhibition and zone of inhibition, are presented in Table 6. The macrocyclic complex [Sn(ML2)Cl2] showed higher antifungal activity with zones of inhibition of 24.1 mm against A. niger and25.3 mm against F. oxysporum at 200 ppm (Fig. 5).
Table 5: Antibacterial activity of Ligands and their tin(II) complexes
|
Compounds |
Bacterial Inhibition Zone (in mm), Concentration (ppm) |
|||||
|
S. aureus |
E. coli |
X. Campestris |
||||
|
500 |
1000 |
500 |
1000 |
500 |
1000 |
|
|
ML1 |
15.3 |
17.4 |
10.9 |
18.9 |
12.4 |
19.5 |
|
ML2 |
17.4 |
20.4 |
12.8 |
21.8 |
15.6 |
22.4 |
|
[Sn(ML1)Cl2] |
20.8 |
23.8 |
15.9 |
25.4 |
16.4 |
26.7 |
|
[Sn(ML2)Cl2] |
21.6 |
24.5 |
17.0 |
26.1 |
20.5 |
27.1 |
|
Gentamicin (Standard) |
22.7 |
25.9 |
17.4 |
26.9 |
21.0 |
27.6 |
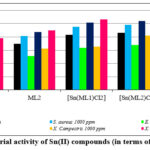 |
Figure 4: Antibacterial activity of Sn(II) compounds (in terms of zone of inhibition). |
Table 6: Antifungal activity of Ligands and their Sn(II) complexes.
|
Compound |
Fungal inhibition Zone (in mm), Conc. (ppm) |
|||||
|
A. niger |
F. oxysporum |
|||||
|
50 |
100 |
200 |
50 |
100 |
200 |
|
|
ML1 |
11.5 |
12.8 |
15.4 |
12.1 |
13.0 |
16.0 |
|
ML2 |
14.3 |
15.6 |
17.9 |
14.6 |
16.4 |
18.2 |
|
[Sn(ML1)Cl2] |
16.9 |
20.2 |
22.6 |
17.2 |
21.0 |
22.8 |
|
[Sn(ML2)Cl2] |
18.0 |
21.2 |
24.1 |
18.9 |
22.1 |
25.3 |
|
Bavistin (Standard) |
18.4 |
21.8 |
24.3 |
19.5 |
22.4 |
25.9 |
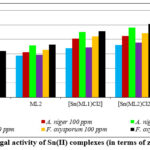 |
Figure 5: Antifungal activity of Sn(II) complexes (in terms of zone of inhibition). |
Brine Shrimp Lethality
The (B.S.L.) Brine Shrimp Lethality of all the compounds was determined, and the LC50 values for each compound have been premediated and are offered. Mortality rate of the nauplii increased as concentration of each sample increased. When plotting the graph of sample’s concentration against %age of mortality, a linear correlation was observed (Figure 6). From this graph, LC50 values for the samples were determined and found to be 28.91, 15.31, 9.07, and 6.01 µg/mL, respectively.
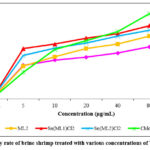 |
Figure 6: Mortality rate of brine shrimp treated with various concentrations of Tin(II) compounds. |
The effectiveness of a drug relies on factors such as its size, shape, and degree of ionization. Biological activity is not solely determined by a single functional group; rather, it often depends on multiple functional groups. Therefore, the addition of any (FG) functional group to an inactive organic substance does not usually confer specific biological activity, as potent activity typically requires more than one functional group. The standard drug chloramphenicol has LC50 value 18.20 µg/mL. The LC50 values are used to infer which concentration is required to show a toxic effect on humans. The lower the LC50 value, the more toxic the compound. Based on this observation, it is concluded that compounds ML1 and ML2 have relatively low cytotoxic activity compared to the compounds [Sn(ML1)Cl2] and [Sn(ML2)Cl2]. The macrocyclic compound ML1 is less toxic than standard drug chloramphenicol. Between ML1 and ML2, ML1 exhibited even less activity than ML2. Similarly, a comparison between compounds [Sn(ML1)Cl2] and [Sn(ML2)Cl2] revealed that [Sn(ML2)Cl2] is more active than the former compound. This suggests that the inclusion of glutaric acid in the compound [Sn(ML2)Cl2] renders it extremely contaminated for brine shrimp nauplii (B.S.N.). While the compound [Sn(ML2)Cl2] displayed significant antimicrobial activity, it exhibited less cytotoxicity, indicating its potential as a safe and effective antibiotic. However, further comprehensive investigations on higher animal models are necessary to study its other potential toxic effects.
Conclusion
In this study, the new ligands and their chelates of Sn(II) metal ions were synthesized. Their structures i.e. octahedral were investigated utilising spectroscopic and elemental studies. The in vitro testing of the free ligand and its metal chelates against two different types of bacteria demonstrated their remarkable efficacy. In addition, Sn(II) complex appears to be a strong option, exhibiting concurrent antioxidant and anticancer effects based on comparative studies, providing a reliable substitute for traditional chemotherapy medicines. The compound [Sn(ML2)Cl2] has the potential to be a safe and effective antibiotic due to its considerable antibacterial activity and low cytotoxicity.
Acknowledgement
The author (Mamta and Subhash) expresses sincere gratitude to the University Grants Commission, New Delhi, India, for the generous financial support received in the form of a Junior Research Fellowship (JRF).
Conflict of Interests
The authors declare that they have no conflict of interests regarding the publication of this article.
Funding Sources
There is no funding sources.
References
- Hrichi, H; Elkanzi, N.A.; Ali A.M.; Abdou A. A novel colorimetric chemosensor based on 2-[(carbamothioylhydrazono) methyl]phenyl 4-methylbenzenesulfonate (CHMPMBS) for the detection of Cu(II) in aqueous medium. Res. Chem. Intermed., 2023, 49, 2257-2276.
CrossRef - Al‐Hamdani, A.A.S.; Al‐Zoubi, W. New metal complexes of N3 tridentate ligand: Synthesis, spectral studies and biological activity. Spectrochim. Acta. Part A: Mole. and Biomol. Spect., 2015, 75, 137.
CrossRef - Arafath M. A.; Adam F.; Ahamed M. B. K.; Karim M. R.; Uddin M. N.; Yamin B. M.; Abdou A. Ni (II), Pd (II) and Pt (II) complexes with SNO-group thiosemicarbazone and DMSO: Synthesis, characterization, DFT, molecular docking and cytotoxicity. J. Mol. Struct., 2023, 1278, 134887.
CrossRef - Samardžija, K.B.; Hackerman, N.; Sovilj, S.P.; Jovanovic, V.M. The inhibiting effect of cobalt(III)-cyclam complexes with substituted β-diketones on iron corrosion in perchlorate solution. J. Solid State Electrochem., 2008, 12, 155-163.
CrossRef - Jovanovic, V.M.; Babic-Samardzija, K.; Sovilj, S.P. Electrochemical examination of mixed-ligand cobalt(III) complexes with tetraazamacrocyclic ligand and heterocyclic dithiocarbamates. Electroanalysis, 2001, 13, 1129-1135.
CrossRef - Paisey, S.J.; Sadler, P.J. Anti-viral cyclam macrocycles: rapid zinc uptake at physiological pH. Chem. Commun., 2004, 3, 306-307.
CrossRef - Haezam, F. N.; Awang, N.; Kamaludin, N. F.; Mohamad, R. Synthesis and Cytotoxic Activity of Organotin(IV) Diallyldithiocarbamate Compounds as Anticancer Agent towards Colon Adenocarcinoma Cells (HT-29). Saudi J. Biol. Sci., 2021, 28(5), 3160–3168.
CrossRef - Maxim, C.; Badea, M.; Rostas, A.M.; Chifiriuc, M.C.; Pircalabioru, G.G.; Avram, S.; Olar, R. Copper(II) species with 1-(o-tolyl)biguanide: Structural characterization, ROS scavenging, antibacterial activity, biocompatibility and in silico studies. Appl. Organomet. Chem., 2022, 36(1), e6471.
CrossRef - Maurer, R.I.; Blower, P.J.; Dilworth, J.R.; Reynolds, C.A.; Zheng, Y.; Mullen G.E.D. Studies on the Mechanism of Hypoxic Selectivity in Copper Bis(Thiosemicarbazone) Radiopharmaceuticals. J. Med. Chem., 2002, 45, 1420-1431.
CrossRef - Cowly, A.R.; Dilworth, J.R.; Donnely, P.S.; Labisbal, E.; Sousa, A. An Unusual Dimeric Structure of a Cu(I) Bis(thiosemicarbazone) Complex: Implications for the Mechanism of Hypoxic Selectivity of the Cu(II) Derivatives. J. Am. Chem. Soc. , 2002, 124, 5270-5271.
CrossRef - Ferrari, M.B.; Bisceglie, F.; Pelosi, G.; Sassi, M.; Tarasconi, P.; Cornia, M.; Capacchi, S.; Albertini, R.; Pinelli, S. Synthesis, characterization and X-ray structures of new antiproliferative and proapoptotic natural aldehyde thiosemicarbazones and their nickel(II) and copper(II) complexes. J. Inorg. Biochem., 2002, 90, 113-126.
CrossRef - Jouad, E.M.; Thanh, X.D.; Bouet, G.; Bonneau, S.; Khan, M.A. In vitro and in vivo effects of [Ni(M5FTSC)2Cl2] complex in cancer: preliminary tests. Anticancer Res., 2002, 22, 1713-1716.
- Chaudhary, A.; Rawat, E. Macrocyclic Assembly: A Dive into the Pecking Order and Applied Aspects of Multitalented Metallomacrocycles. Int. J. Inorg. Chem., 2014, Volume 2014, 1-30.
CrossRef - Subhash; Jyoti; Chaudhary, A. Synthesis, spectroscopic characterization, in vitro cytotoxic, antimicrobial and antioxidant studies of Co(II) complexes bearing pyridine-based macrocyclic ligands with density function theory (DFT) and molecular docking investigations. Res Chem Intermed 2023, 49, 4729-4758.
CrossRef - Javed, F.; Sirajuddin, M.; Ali, S.; Khalid, N.; Tahir, M. N.; Shah, N. A.; Rasheed, Z.; Khan, M. R. Organotin(IV) Derivatives of o-Isobutyl Carbonodithioate: Synthesis, Spectroscopic Characterization, X-Ray Structure, HOMO/LUMO and In vitro Biological Activities. Polyhedron, 2016, 104, 80–90.
CrossRef - Chaudhary, A.; Rawat, E.; Khan, S.; Jindal, P.; Singh, R.V. Synthesis, Spectroscopic Characterization, Antimicrobial, Pesticidal and in Vitro Antitumor Activity of Macrocyclic Complexes of Tin(II). Chem. Sci. Rev. Lett., 2014, 3, 225-240.
- Chaudhary, A.; Rawat, E.; Khan, S. A New Class of 16 To 22-Membered Tetraazatetraoxo Macrocyclic Complexes of Tin(II): Synthesis, Structural Elucidation and Fungicidal Properties. Int. J. Res. Eng., 2013, 1(3), 1.
- Subhash; Chaudhary, A., Jyoti et al. Synthesis, structural elucidation, DFT investigations, biological evaluation and molecular docking studies of tetraamide-based macrocyclic cobalt (II) complexes. J Iran Chem Soc 2023, 20, 2339-2362.
CrossRef - Chaudhary, A.; Rawat, E.; Khan, S. Repellent Action of Macrocyclic Complexes of Tin(II) Against Adult Khapra Beetle, Trogoderma Granarium (Everls) Infesting Wheat Grain. Int. J. Chem. Chem. Eng. Syst., 2013, 3, 2248.
- Chaudhary, A.; Singh A. Macrocyclic Complexes: A New Way forward into the Medicinal World, Int. J. Adv. Res, 2016, 4(9), 1004-1015.
CrossRef - Subhash; Chaudhary, A; Mamta; Jyoti. Synthesis, structural characterization, thermal analysis, DFT, biocidal evaluation and molecular docking studies of amide-based Co(II) complexes. Chem. Pap. 2023, 77, 5059-5078.
CrossRef - Mamta; Subhash; Pinki; Chaudhary, A. In Vitro Cytotoxicity and Antimicrobial Evaluation of Novel 24-28 Membered Schiff Base Octaazamacrocyclic Complexes of Manganese (II): Synthesis, Characterization, DFT and Molecular Docking Studies, J. Mol. Struct., 2023, 1275, 134667.
CrossRef - Pinki; Mamta; Chaudhary, A. Synthesis, spectroscopic elucidation, in vitro antimicrobial, cytotoxic and CT-DNA binding evaluation of heterobimetallic complexes of Ni(II) with main group/transition metal dichlorides, J. Mol. Struct., 2023, 1279, 134936.
CrossRef - Mamta; Pinki; Subhash; Chaudhary A. Synthesis, spectroscopic characterization, density functional theory, molecular docking studies, antioxidant and antimicrobial potential of novel Schiff base macrocyclic complexes of bivalent manganese, Appl. Organomet. Chem., 2023, 37 (6), e7095.
CrossRef - Subhash; Chaudhary, A.; Jyoti, Kumar, M.; Kumar, N.; Agarwal, N.K. Synthesis, Spectroscopic Characterization, Biocidal evaluation Molecular Docking & DFT Investigation of 16-18 membered Macrocyclic Complexes of Cobalt (II). J. Chem. Sci., 2022, 134, 113.
CrossRef - Sharma, K.; Joshi, S.C.; Singh, R.V. Fertility inhibitor heterobimetallic complexes of platinum (II) and palladium (II): synthetic, spectroscopic and antimicrobial aspects. Metal Based Drugs, 2000, 7, 105.
CrossRef - Pellerito, C.; Nagy, L.; Pellerito, L.; Szorcsik, A. Biological Activity Studies on Organotin(IV) N+ Complexes and Parent Compounds. J. Organomet. Chem., 2006, 691(8), 1733-1747.
CrossRef - Chaudhary, A.; Singh, A.K.; Singh, R.V. Investigations of the Possible Pharmacological Effects of Organotin (II) Complexes. J. Inorg. Biochem., 2006, 100,1632.
CrossRef - Shakir, M.; Varkey, S.P. A new synthetic route for the preparation of a new series of 14–22-membered tetraoxomacrocyclic tetraamines and their transition metal complexes. Polyhedron, 1995, 14, 1117.
CrossRef - Bansal, A.; Fahmi, N.; Singh, R.V. Synthesis And Spectral Studies On New 12- And 13- Membered Tetraza Macrocyclic Complexes of Tin and Lead. Main Group Met. Chem., 1999, 22, 111.
CrossRef - Singh, H.L. Synthesis, spectroscopic, and theoretical studies of tin(II) complexes with biologically active Schiff bases derived from amino acids. Main Group Met. Chem., 2016, 39(3-4): 67–76.
CrossRef

This work is licensed under a Creative Commons Attribution 4.0 International License.









