Ethnomedicinal and Phytochemical Studies of Eclipta alba (A Review)
1School of Pharmaceutical Sciences, IFTM University, Lodhipur Rajput, Moradabad, Uttar Pradesh, India.
2Faculty of Pharmacy, IFTM University, Lodhipur Rajput, Moradabad, Uttar Pradesh, India.
Corresponding Author E-mail: srishtigoyal.sg28@gmail.com
DOI : http://dx.doi.org/10.13005/ojc/400101
Article Received on : 26 Oct 2023
Article Accepted on : 08 Jan 2024
Article Published : 16 Jan 2024
Reviewed by: Dr. Mudit Sharma
Second Review by: Dr. Lamia Sebaa
Final Approval by: Dr.Kasthuri Pandian,
Traditional plants have tremendous benefits for many ailments and illnesses that are typically inexpensive and side-effect-free. We highlight in this review comparatively extensive data on the ethnomedicinal applications, phytochemistry, pharmacology, and toxicity of Eclipta alba. The scientific data was gathered from books and online bibliographic sources like PubMed, Google Scholar, SciFinder, and Scopus. The phytochemical analysis of this plant has afforded an important category of natural products such as coumestans, terpenoids, alkaloids, volatile oils, flavonoids, and thiopenes. According to reports, coumestans are the most often used components among them. Potential pharmacological effects, including wound healing, diabetes, obesity, antioxidants, cancer, hair growth, and neuroprotective properties, have been reported for the extracted crude extract and independent component. Subsequent research ought to concentrate on in-depth mechanism-based investigations using clinical trials and animal models.
KEYWORDS:Eclipta alba; Ethnomedicinal; Phytochemicals studies; Pharmacological activity; Toxicology
Download this article as:| Copy the following to cite this article: Goyal S, Mani M, Kumar P. Ethnomedicinal and Phytochemical Studies of Eclipta alba (A Review). Orient J Chem 2024;40(1). |
| Copy the following to cite this URL: Goyal S, Mani M, Kumar P. Ethnomedicinal and Phytochemical Studies of Eclipta alba (A Review). Orient J Chem 2024;40(1). Available from: https://bit.ly/4b13bA7 |
Introduction
Natural-origin drugs have a significant role in the healthcare system. The oldest system of traditional medicine is Ayurveda. Ayurvedic practices use formulations made from around 600 different medicinal herbs to address a variety of diseases1. Eclipta alba, syn. Eclipta prostrata is a kind of small perennial herbaceous plant grown in tropical areas worldwide. Other than this it’s also evenly distributed in China, India, Brazil, Thailand, Sri Lanka, Malaysia, and Nepal with moist soil, watercourse, and hilly areas2,3.
As it is known for its traditional use in various ailments. It’s commonly known as bhringraj, false daisy. Eclipta alba is mainly of three in variety i.e., the white flowering, the black fruiting, and the yellow flowering and all are grown all over India on the sides of rivers, lakes, and foothills of mountains4,5,6. In Traditional Chinese Medicine the dried aerial portion of the plant is known as yin-nourishing herb. They are frequently used to treat associated conditions like hemorrhage, greying hair, tinnitus, and dizziness7.
Botanical characterization of Eclipta alba
Eclipta alba is an annual herb growing up to 30-40cm tall, erect, or sometimes rooting at the nodes8. The stem has fine hairs on its surface, making it rough and either flat or cylindrical, with branched nodes blackish-green in color. Roots are cylindrical and gray in color. The leaves are sessile to sub-sessile, opposite 1.2-2.3 cm wide and 2.2-8.5 cm long oblong, lanceolate, strigose with fine hairs on both sides of the surface9,10,11. The flowers are white, small, and arranged in a tiny bundle. The leaf axis gives rise to the flowering stalk, the inflorescence is racemose, and the bloom can be actinomorphic, zygomorphic, pentamerous, or unisexual. Androecium with 5 stamens, an epipetalous filament, a free anther, and an obtuse base, gynoecium with two carpels fused formed the fruit, Ovary inferior, unilocular with 1 basal ovule. Fruit is achenes cuneate, pappus, one seeded with slight wing and brown in appearance. Seeds are dark brown, hairy outer layer, height 0.2-0.25cm and width 0.1cm, non-endospermic dicotyledon12.
 |
Figure 1: Picture of the plant Eclipta alba. |
Taxonomy of Eclipta alba13
Kingdom Plantae
Sub-kingdom Viridaeplantae
Division Tracheophyta
Sub-division Spermatophytina
Class Magnoliopsida
Order Asterales
Family Asteraceae
Genus Eclipta
Species alba
Various vernacular names of Eclipta alba in distinct language, common name is Bhringraj, False Daisy; in Hindi bhangara, bhangaraiya; in Sanskrit keshraj, markava, bhrunga; in Bengali kesuriya, kesari; in Gujrati bhangaro; in Urdu bhangra; in Tamil karisalai; in Marathi maka; in Telegu guntagalagara; in Kannada garujalu, soppu, gurugada; in Malayalam kayyonni, knnunni; in Oriya kesara, kesarda; in Punjabi bhangra; in Assamese bhringraja; in Arabic kadimulabit 14,15.
Ethnomedicinal uses of Eclipta alba
In several parts of India, the plant Eclipta alba is used to treat conditions like respiratory illnesses, gastrointestinal problems, along skin diseases. The leaves of plants are used in the cure of hair fall, hair drying, alopecia16, gingivitis, diabetes, baldness, headache17, elephantiasis, hair dye and stem used as a blood tonic, anemia, any other blood-related problems18, chickenpox19 as well as its roots are having great result with constipation & irregular bowels20. The whole plant is used in the prevention of symptoms like burns, sores, asthma, cuts and wounds22, ulcers, fever, normal weakness, jaundice, liver-related, snake bite21, edema, swelling, high BP, diabetes, indigestion, hepatitis25, spleen enlargement, anticatarrhal, febrifuge fetal development & childbirth facilitates23,24. The aerial portion of Eclipta alba is taken as a hepatic cleanser, and hair tonic26, allergic, inflammation, burns, skin disorder, anemia, headache27, mental disorder, astringent, antiseptic, anticancer28,29. There are numerous Ayurvedic formulations present from the old times, a few of them are; for hair fall (Khalitya) Bhringrajtaila, Gujadi oil30,34, tonic for hair growth, graying or complexation of hair Bhringrajadichurana31, Bhringrajghrit32, Bhringrajasasv33, and for improving the density Bhringrajchurna is used35 36.
Table 1: Phytocompounds derived from Eclipta alba
|
No. |
Compounds |
Part |
References |
|
Coumestans 1 2 3 4 |
Wedelolactonea Demethylwedelolactone-7-glucosideb Iso-demethylewedelolactonec Strychnolactone |
Aerial Aerial Whole Plant Aerial |
37,38,39 39,40 40 40 |
|
Terpenoids, their glycosides (taraxastane triterpene glycoside) 5 6 7 8 (Oleanane triterpene glycosides) 9 10 11 12 13 14 15 16 17 18 19 20 21 22 23 24 25 26 27 28 29 30 31 32 33 34 Alkaloids 35 36
37 38 39 40
41 42 Volatile Oils 43 44 45 46 47
48
49
50 51 52 53 54 |
Eclabasaponins Ⅶ Eclabasaponins Ⅷ Eclabasaponins Ⅸ Eclabasaponins Ⅹd Eclabasaponins Ⅰe Eclabasaponins Ⅱf Eclabasaponins Ⅲg Eclabasaponins Ⅳh Eclabasaponins Ⅴi Eclabasaponins Ⅵj Ecliptasaponin Ak Ecliptasaponin B Ecliptasaponin C Ecliptasaponin Dl Eclalbatin a-amyrinm Oleanolic acidn Echinocystic acido Ursolic acidp Stigmasterolq Stigmasterol-3-O-glucosider Daucosterols Silphioside C Silphioside B Silphioside E b-Sitosterolt Machaeroceric acid b-amyrinu Echinocystic acid-28-O-b-D-glucopyranoside Ecliptine (20S) (25S)-22,26-imino-cholesta-5 22(N)-dien-3b-ol (verazine) 25b-hydroxyverazinev 4b-hydroxyverazinew (20R)- 4b-hydroxyverazine Ecliptalbine [(20R)-2-pyridyl-cholesta 5-ene-3P, 23-diol] (20R)-25b-hydroxyverazine (20-epi-3-dehydroxy-3-oxo- 5,6-dihydro-4,5-dehydroverazine) Heptadecanex n-hexadecenoic acid pentadecane, eudesma-4(14) 6,10,14-trimethyl-2-pentadecanone 1,2-benzenedicarboxylic acid diisooctyl ester (Z)-7,11-dimethyl 3-methylene-1,6,10-dodecatriene (Z, Z, Z)-1,5,9,9-tetramethyl-1,4,7-cycloundecatriene Phytol, octadic-9-enoic acid D-dithienyl acetylene ester a-terthienyl-methanoly a-formylterthienyl ecliptal or a-terthienyl aldehyde |
Whole Plant Whole Plant Whole Plant Whole Plant Whole Plant Whole Plant Whole Plant Whole Plant Whole Plant Whole Plant Whole Plant Whole Plant Whole Plant Whole Plant Whole Plant Aerial Whole Plant Whole Plant Whole Plant Leaves/Stem Aerial/ stem Leaves/stem Whole Plant Aerial Aerial Aerial Aerial Aerial Aerial Whole Plant Aerial Leaves Leaves Leaves Whole Plant Leaves Leaves Aerial Aerial Aerial Aerial Aerial Aerial Aerial Aerial Aerial Aerial Aerial Aerial |
41 41 41 41
41,42,43 41,42,43 41,42,43 42,43 41,42,43 42,43 41 41 41 41 45,46 46 46 41 46 44 42,44,47 44 48 49 49 39 42 46 50
41 51
51 51 51 51
51
51
46 46 46 47 46
47
46,47
45 51 51,52,53 51,52,53 51,52,53 |
|
Flavonoids 55 56 57 58 59 60 61 62 |
Protocatechuic acid 4-hydroxybenzoic acid Apigenin Luteolinaa Luteolin-7-O-glucosidebb Pratenseincc Diosmetindd Buddleoside |
Leaves/Whole Plant Leaves/Stem Aerial Aerial Aerial Aerial Aerial Aerial |
39.42,44,46 39,44,46 39,44,46 55 56 48,57,58 58 58 |
|
63 |
Quercetinee |
Aerial |
59,60 |
|
64 65 66 |
Skullcapflavone Ⅱff Kaempferolgg Eriodictyolhh |
Whole Plant Whole Plant Whole Plant |
60 60 48 |
|
67 |
Orobolii |
Whole Plant |
42 |
|
68 |
Acacetinjj |
Whole Plant |
60 |
|
69 70 71 72 73 74 75 76 77 |
Vanillic acidkk Syringic acidll Chlorogenic acidmm Leonuriside Ann Caffeic acidoo Coniferyladehyde Oroboside Orobol-5-O-b-D-glucopyranoside 3’-O-methylorobolpp |
Aerial Aerial Aerial Whole Plant Whole Plant Whole Plant Whole Plant Whole Plant Aerial |
42 42 42 48 48 48 59 48 42,59 |
|
78 |
3’-Hydroxybiochanin A |
Aerial |
57,58 |
|
79 |
Tricetinqq |
Aerial |
42 |
|
Thiophene 80 81 82 83 84 85 86 87
Miscellaneous 88 89 90 91
92
93
94 95 96 97 98 99 100
|
Ecliprostin A, B, C a-Formylterthienyl Arctinol Brr 6-Methoxy-arctinol-b a- Terthienyl a-Terthienyl methanol 2,2’,5”-terthiophene-5-carboxylic acid 5-aldehyde-5’-(3-butene-1-ynyl)-2,2’-dithiophene Heptacosanolss Hentriacontanoltt (Fatty Alcohol) Ecliptal 5-hydroxymethyl-(2,2’:5’,2”)-terthienyltiglate 5-hydroxymethyl-(2,2’:5’,2”)-terthienylagelate 5-hydroxymethyl-(2,2’:5’,2”)-terthienyl acetate Polyacetylenes (Polyacetylinic) Polyacetylene-substituted thiophenes 4-hydroxy benzoic acid (Phenolic acids) Protocatechuic acid (Phenolic acids) Euodionoside A Junipeionoloside Calaliuiuenside |
Aerial Whole Plant Aerial Aerial Aerial Whole Plant Leaves Whole Plant
Roots/Leaves Roots Roots Roots/Leaves
Whole Plant
Whole Plant
Roots Leaves/Whole Plant
Leaves/Stem Whole Plant Whole Plant Whole Plant |
60 53 63 63 52 52,53,54 62 53,54
52,54 52,54 52,54 52,54
52,54
52,54
44,46 44,46 44,46
47 48 48 48 |
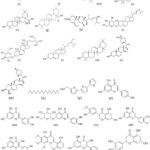 |
Figure 2: Chemical structures of some phytocompounds of Eclipta alba. |
Pharmacological Activities
Wound healing activity
According to this investigated study they created a foam dressing with gelatin and leaf extract from Eclipta prostrata. Utilizing scanning electron microscopy (SEM), the pore structure was discovered and to verify the chemical arrangement, Fourier-transform infrared spectroscopy (FTIR) was utilized. As for the conclusion, it was determined that the Eclipta prostrata dressings had the proper pore size. In the first hour, the weight rise percentage for the Eclipta prostrata B dressings was higher, and in the first four hours, the rate of dehydration was higher62. In another study of wound healing activity with an aqueous extract of Eclipta alba leaves tends to lower the glycemic action in streptozotocin-induced rats. Also, in comparison to cicatryl (J16) and vaselin (J18) Ointment A (aqueous) and B (hydroethanolic) completely healed in J14 wound in animals. The aqueous extract reduces the glycemic action as well as in streptozotocin rats and ointment of aqueous and hydroethanolic extracts was used to treat incision wounds63. Eclipta alba hydroalcoholic and methanolic extract gel induced in diabetes alloxan rats, and gel applied on wounds for 15 days that tended to result in hydroalcoholic extract gel is a more potent action than methanolic & it is non-toxic when taken orally in LD50 rats. Hydroalcoholic gel extract of Eclipta alba has shown the diabetic wound healing action better than methanolic extract64.
Anti-diabetic Activity
As per the reported study, antidiabetic activity of Eclipta prostrata hydro extract in Streptozotocin-induced diabetic rat, oral treatment of Eclipta prostrata and glibenclamide results in efficient in diabetes, improving carbohydrate metabolizing enzymes, also enhance serum high-density lipoprotein cholesterol range65Diabetes is linked to insulin regulation and pancreatic beta-cell progression. Phytonanotherapy uses metal nanoparticles and plants to treat diabetes. This study focuses on the eco-friendly, Eclipta alba (EA-AuNPs) is used in the manufacture of gold nanoparticles, and their pharmacological effectiveness against pathogenic bacteria and streptozotocin-induced apoptosis in the RIN-5F cell line assessed. The synthesized EA-AuNPs showed anti-apoptotic potential and free radical scavenging potential, making them promising for biomedical applications in nanomedicine66 .
Anti-Cancer Activity
The MTT assay experiment in the cancer cell investigation demonstrated a significant (p 0.005) selection against HCT-116 cells by the methanolic extract of Eclipta alba. Additionally, this extract had a negligible or harmless effect on WI-38 cells. Clonogenic and migration assays both supported the effectiveness of the extract’s antitumor activity against HCT-116 cells. The outcomes showed that Eclipta alba methanolic extract has optimal properties, such as little toxicity against normal cells WI-38, and possesses high action against colorectal cancer cells HCT-11667.
Anti-Fungal Activity
Boregowda et al., 2019 reported the antifungal activity against the sorghum fungal pathogens Fusarium thapsinum, Epicoccum sotghinum, Alternaria alternata, and Curvilaria lunata. Methanolic extract of Eclipta alba tested on all pathogenic fungi. The existence of their plant analogs is shown by the chemical characterization of the metabolites, for example, wedelolactone, and eclalbasaponin II are available in huge amounts. To detect the phytochemicals, present in methanolic extract obtained from the plant are determined by Ultra-performance liquid chromatography and mass chromatography68.
Anti-Obesity Activity
On anti-obesity rats with high-fat diets including cholesterol and cholic acids to induce non-alcoholic fatty liver, the effects of Eclipta prostrata methanol extract on this condition were assessed. The lipid profile and liver function significantly improved after increased dose treatment with Eclipta prostrata (200mg/ and 300mg/kg), as per the outcome of biochemical and histological study69.
Anti-inflammatory Activity
Eclipta prostrata improved atopic dermatitis symptoms progression, reduced the layer thickness of the epidermis and dermis, infiltrated defense cells, and healed skin barrier malfunction. Additionally, by restoring the skin barrier and balancing the defense system, it reduces allergic irritation. In HaCat keratinocytes, Eclipta prostrata suppressed nuclear factor-kB translocation, phosphorylation, and cytokine expression, indicating promising therapeutic use as an anti-atopic drug70.
Immunomodulatory Effect
A study on heteropneustes fossilis fingerlings found that Eclipta alba extract, at a concentration between 50-100ppm, protects them against A. invadans infection. The extract induces anti-stress and antioxidative responses, reducing cortisol levels and increasing superoxide dismutase and catalase levels. The protective effect is linked to its immunomodulatory effect, enhancing fingerling survival. Eclipta alba extract could be a potential holistic strategy for controlling EUS in fish Species71.
Antioxidant Activity
The study looked into the antiproliferative effects, antioxidant ability, and chemistry of extracts from Eclipta prostrata. Two flavonoids were extracted from the ethyl acetate extract: 3-O-methylorobol and apigenin 7-sulfate. Compared to other extracts, the ethyl acetate extract exhibited larger phenol and flavonoid levels as well as stronger antioxidant activity. It decreased AGS cell viability and proliferation in vitro by changing gene expression to cause apoptosis72. Research on Eclipta alba found that exposure to multispectral lights significantly enhanced the growth and development of the plant. Red light led to maximum dry weight and increased phenolics and flavonoid content in callus cultures. HPLC analysis the highest accumulation of major compounds in red light-treated cultures, while blue light led to the optimal accumulation of stigmasterol. These findings suggest that multispectral lights can be an effective strategy for enhancing phytochemical production in Eclipta alba 73.
Nephroprotective Activity
Dacus carota and Eclipta prostrata extract nephroprotective properties on Wistar albino rats’ nephrotoxicity produced by cisplatin. Four groups of rats were created, and samples of their urine and blood were taken. The findings of cisplatin-induced nephrotoxicity in rats showed that Cis + DC/Cis + EP (600mg/kg) markedly raised body weight and decreased kidney weight. It also raised the amount of Na, and K, creatinine in the urine and plasmin74.
Toxicology
The 70% ethanol fraction lethal dose (LD50) in a mouse trail of acute toxicity was found to be undetectable, as no animals died even after being given a dose of 10.4 g/kg for 14 days61. Bone marrow stromal cells were cytotoxically affected by high doses of wedelolactone (10mmol/L) and ethyl acetate extract (20mg/mL). Additionally, a dose greater than 40mmol/L of wedelolactone inhibited human renal mesangial cell proliferation. The incongruous result differing solvents extract, ingredients, an experimental model’s studies may be the reason, indicating that extra care is still required for the dosage to ensure safety 75.
Conclusion
The plant Eclipta alba is widely used in traditional medicine worldwide, especially for conditions pertaining to the stomach, liver, and skin. It is also used to promote hair development. It has a variety of phytochemical components, including coumestans, saponins, alkaloids, polyacetyl, wedelolactone, eclalbasaponins, a-amyrin, ursolic acid, oleanolic acid, luteolin, and apigenin are among the substances found in flavonoids, which have biological effects on the body, including hepatoprotective, anti-depressant, anti-inflammatory, antibacterial, and antidiabetic effects. Eclipta alba extract may prove to be a valuable source for the pharmaceutical and nutraceutical industries in the future if establishing standards and stability studies are conducted on it. By gathering information from pharmaceutical and clinical studies, the lead bioactive compounds could be further developed as therapeutic molecules.
Acknowledgment
The authors are highly thankful to IFTM University for providing the necessary facilities for the review work.
Conflict of Interest
The authors declare no conflict of interest.
References
- Singh, AP. Ethnobotanical leaflets 2005, (1), 18.
- Jadhav, V.M.; Thorat, R.M.; Kadam, V. J.; Salaskar, K. P. Journal of Pharmacy Research 2009, 2(8), 1129-1231.
- Mahmood, S.; Hussain, S.; Malik, F. Pakistan journal of pharmaceutical sciences. 2013, (26), 6.
- Kapoor, L.D. CRC Press LLC. 2001, 169.
- Treadway, S. Clin Nutr Insights. 1998, 6(16), 1-3.
- Parrey, M.S.; Ahmad, I. World Journal of Pharmacy and Pharmaceutical Sciences. 2016, 5(12), 504-512.
- Chinese Pharmacopoeia Commission. China Medical Science Publisher. 2015, 1, 374-375.
- Feng, L.; Zhai, Y.Y.; Xu, J.; Yao, W.F.; Cao, Y.D.; Cheng, F.F.; Bao, B.H.; Zhang, L. Journal of Ethnopharmacology. 2019, 245, 112109.
- Ministry of Health and Family Welfare. Pharmacopoeia Commission for Indian Medicine & Homeopathy. 1999, 21-22.
- Kumar, S. Phytochemistry. 2019, (9), 10.
- Chung, I.M.; Rajakumar, G.; Lee, J.H.; Kim, S.H.; Thiruvengadam, M. Applied microbiology and biotechnology. 2017,101, 5247-5257.
- Neeraja, P.V..; Margaret. E. Int J Curr Pharmaceut Rev Res. 2012, 2(4), 188-197.
- Mukhopadhyay, G.; Kundu, S.; Sarkar, A.; Sarkar, P.; Sengupta, R.; Kuamr, C. Pharm. Innov. J. 2018, 7(9), 78-83.
- Patle, K.; Kumar, N.; Sahu, S.; Sahu, U.; Komre, P.; Sahu, R.; Banafar, A.; Dewangan K.P.; Nayak, P.; Patel, P.; Nagori, K.; Sharma, M. Eur. Chem. Bull. 2023, 12(5), 2053-2070.
- Khare, C.P. Springer-Verlag Berlin Heidelberg. 2007, 230-231.
- Sharma, T.; Yusuf, M.; Hussain, S.; Abrar, H. Int. Res. J. Pharm, 2012, 3, 51-53.
- Khan, A.V.; Khan, A.A. Indian Journal of Traditional Knowledge, 2008, 7(2), 316-320.
- Parveen, U.B.; Roy, S.; Kumar, A. Ethnopharmacology. 2007, 113(3), 387-399.
- Rahmatullah, M.; Mollik, M.A.; Azam, A.T.; Islam, M.R.; Chowdhury, M.A.; Jahan, R.; Chowdhury, M.H.; Rahman, T. American Eurasian Journal of Sustainable Agriculture, 2009, 3(4), 889-898.
- Jeyaprakash, K.; Ayyanar, M.; Geetha, K.N.; Sekar, T. Asian Pacific Journal of Tropical Biomedicine. 2011, 1(1), S20-5.
- Gautam, A.; Batra, A. World Journal of Pharmaceutical Sciences, 2012, 1(1), 10-18.
- Korpenwar, A.N. International Journal of Recent Trends in Science and Technology. 2012, 3, 49-53.
- Umesh, A.; Kumudhavalli, M.V. International Journal of Research in Pharmacy and Chemistry, 2020, 10(1), 63-70.
- Rafif, K.A.; Intan, S.T.; Muhammad, A.A.; Mulyo, R.H. Research Journal of Biotechnology. 2022 17, 3.
- Thakur, V.D.; Mengi, S.A. J Ethnopharmacol, 2005, 102(1), 23-31.
- Roy, R.K.; Thakur, M.; Dixit, V.K. Archives of Dermatological Research. 2008, 300(7), 357-364.
- Nadkarni, K.M.; Nadkarni, A.K. Popular Prakashan. 1994, 1, 471-472.
- Xi, Q.J.; J. China Prescr. Drug. 2018, 16(8), 15-17.
- Li, W.; Pang, X.; Han, L.F.; Zhou, Y.; Cui, Y.M. China Journal of Chinese Materia Medica. 2018, 43(17), 3498-3505.
- Jadhav, V.M.; Thorat, R.M.; Kadam, V.J.; Gholve, S.B. International Journal of Pharmtech Research. 2009, 1(3), 454-467.
- Saraswat, V.K.; Verma, S.; Musale, S.V.; Jaiswal, M.L. International Ayurvedic Medical Journal. 2015, 3(8), 2462-2469.
- Charpe, T.W.; Rathode, V.K. Brazilian Journal of Chemical Engineering. 2016, 33, 1003-1010.
- Alam, M.K.; Tuli, K.A.; Khan, M.K.I.; Hossain, M.S. World journal of pharmaceutical and life sciences. 2022, 8(4), 133-136.
- Pathak, A.P.; Misar, S. Int Ayurveda Public, 2017, 2, 675-680.
- Pandey, M.K.; Singh, G.N.; Sharma, R.K.; Lata, S. International journal of pharmaceutical sciences and research. 2012, 3(1), 171.
- Gautam, T.P. Nepalese Journal of Biosciences. 2011, 1, 125-130.
- Kaushik, B.N.; Bopda, W.A.; Talele, T.T.; Basu, A.; Costa, P.R.; Da Silva, A.J.; Sarafianos, S.G.; Noel, F. Nucleic acids research. 2008, 36(5), 1482-1496.
- Zhang, J.S.; Guo, Q.M. Acta Pharmaceutica Sinica. 2001, 36(1): 34-37.
- Yahara, S.; Ding, N.; Nohara, T.; Masuda, K.; Ageta, H. Phytochemistry. 1997, 44, 131-135.
- Le, D.D.; Nguyen, D.H.; Ma, E.S.; Lee, J.H.; Min, B.S.; Choi, J.S.; Woo, M.H. Biological and pharmaceutical bulletin. 2021, 44(3), 298-304.
- Yu, S.J.; Yu, J.H.; Yu, Z.P.; Yan, X.; Zhang, J.S.; Sun, J.Y.; Zhang, H. Phytochemistry. 2020, 170, 112192.
- Zhang, M.; Chen, Y.Y.; Di, X.H.; Liu, M. Acta Pharmaceutica Sinica. 1997, 32(8), 633-634.
- Khanna, V.G.; Kannabiran, K. International Journal of Green Pharmacy. 2009, 1, 227-229.
- Upadhyay, R.K.; Pandey, M.B.; Jha, R.N.; Pandey, V.B. Journal of Asian Natural Products Research. 2001, 3(3), 213-217.
- Jahan, R.; Al-Nahain, A.; Majumdar, S.; Rahmatullah, M. International Scholarly Research Notices. 2014, 1-22.
- Xiong, H.P.; Xi, F.M.; Chen, W.S.; Lu, W.Q.; Wu, Z.J. Chemistry of natural compounds. 2021, 57, 166-168.
- Xi, F.M.; Li, C.T.; Han, J.; Yu, S.S.; Wu, Z.J.; Chen, W.S. Bioorganic and Medicinal Chemistry. 2014, 22(22), 6515-6522.
- Han, L.; Zhao, J.; Zhang, Y.; Kojo, A.; Liu, E.; Wang, T. Chinese Herbal Medicines. 2013, 5(4), 313-316.
- Abdel-Kader, M.S.; Bahler, B.D.; Malone, S.; Werkhoven, M.C.; Van, T.F.; Wisse, D.J.; Bursuker, I.; Neddermann, K.M.; Mamer, S.W.; Kingston, D.G. Journal of Natural Products. 2000, 63(8), 1202-1208.
- Kim, D.I.; Lee, S.H.; Choi, J.H.; Lillehoj, H.S.; Yu, M.H.; Lee, G.S. Nutrition research. 2008, 28(8), 550-554.
- Meng, X.; Li, B.B.; Lin, X.; Jiang, Y.Y.; Zhang, L.; Li, H.Z.; Cui, L. Journal of Asian Natural Products Research. 2019, 21(6), 501-506.
- Lee, J.S.; Ahn, J.H.; Cho, Y.J.; Kim, H.Y.; Yang, Y.I.; Lee, K.T.; Jang, D.S.; Choi, J.H. Journal of Ethnopharmacology. 2015. 169, 426-434.
- Kim, H.Y.; Kim, H.M.; Ryu, B.; Lee, J.S.; Choi, J.H.; Jang, D.S. Arch Pharm. Res. 2015, 38(11), 1963-1969.
- Singh, B.; Saxena, A.K.; Chandan, B.K.; Agarwal, S.G.; Anand, K.K. Indian J. Physiol. Pharmacol. 2001, 45(4), 435-441.
- Feng, L.; Zhai, Y.Y.; Xu, J.; Yao, W.F.; Cao, Y.D.; Cheng, F.F.; Bao, B.H.; Zhang, L. Journal of Ethnopharmacology. 2019, 245, 112109.
- Han, L.; Zhao, J.; Zhang, Y.; Kojo, A.; Liu, E.; Wang, T. Herb. Med. 2013, 5, 313-316.
- Timalsina, D.; Devkota, H.P. Biomolecules. 2021, 11(11), 1738.
- Li, W.; Pang, X.; Han, L.F.; Zhou, Y.; Cui, Y.M. China Journal of Chinese Materia Medica. 2018, 43(17), 3498-3505.
- Zhao, Y.; Peng, L.; Lu, W.; Wang, Y.; Huang, X.; Gong, C.; He, L.; Hong, J.; Wu, S.; Jin, X. Experimental Gerontology. 2015, 62, 37-40.
- Yu, S.J.; Yu, J.H.; He, F.; Bao, J.; Zhang, J.S.; Wang, Y.Y.; Zhang, H. Fitoterapia. 2020, 142, 104471.
- Yu, S.J.; Zhang, J.S.; He, H.; Yu, J.H.; Bao, J.; Zhang, H. Journal of Asian Natural Products Research. 2021, 23, 745-753.
- Hasatsri, S.; Suthi, J.; Siriwut, N.; Charoensappakit, O. Pharmaceuticals. 2023, 16(685), 1-14.
- Raoul, A; Jonas, M.C.; Rommelle, S.M.C.; Romaric, E.I.D.G.; Martin, D.; Antoine, A.A. Int. J. Adv. Res. 2018, 6, 393-398.
- Prajapat, A.; Pandey, R. Int. J. of Pharm. Life Sci. 2018, 9 (5,6), 5813-5816.
- Zhang, L.; Zheng, C.; Xu, T.; Du, L. Sains Malaysiana. 2022, 51(10), 3359-3370.
- Kumar, V.S.; Vinayagam, R.; Anand, V.A.M.; Venkatachalam, K.; Kumar, S.K.; Wang, H.M.; Casimeer, S.; K.M, Gothandam.; David, E. J Drug Deliv Sci Technol. 2020, 58, 101786.
- Nelson, V.K.; Sahoo, N.K.; Sahu, M.; Sudhan, H.H.; Pullaiah, C.P.; Muralikrishna, K.S. BMC Complementary medicine and therapies. 2020, 20(1), 1-8.
- Boregowda, R.S.; Murali, N.; Udayashankar, A.C.; Niranjana, S.R.; Lund, O.S.; Prakash, H.S. Plants. 2019, 8(3), 1-16.
- Helmy, A.S.; Sherif, N.M.; Ghanem, H.Z.; Ibrahim, N.A.; EI Gendy, A.N.; Hussein, N.S.; Abdel-Hamid, A.H. Journal of Applied Pharmaceutical Science. 2019, 9(1), 077-90.
- Kang, Y.M.; Kim, H.M.; Lee, H.; Lee, D.S.; An, H.J. J Ethnopharmacol. 2022, 292, 1-11.
- Kumar, V.; Das, B.K.; Swain, H.S.; Chowdhury, H.; Roy, S.; Bera, A.K.; Malick, R.C.; Behera, B.K. J. Fungi. 2023, 9, 142.
- Yang, J.; Kim, J.S.; Kwon, Y.S.; Seong, E.S.; Kim, M.J. Molecules. 2023, 28, 7354.
- Khurshid, R.; Ullah, M.A.; Tungmunnithum, D.; Drouet, S.; Shah, M.; Zaeem, A. PLoS ONE. 2020, 15(6), e0233963.
- Iqbal, M.O.; Sial, A.S.; Akhtar, I.; Naeem, M.; Hazafa, A.; Ansari, R.A.; Rizvi, A.A. Bioengineered. 2021, 12(2), 12702-12721.
- Liu, Y.Q.; Zhan, L.B.; Liu, T.G.; Cheng, M.C.; Liu, X.Y.; Xiao, H.B. J. Ethnopharmacol. 2015, 157, 206-211.

This work is licensed under a Creative Commons Attribution 4.0 International License.










