Dioxomolybdenum (VI) Compounds of Macrocyclic Schiff base Ligands: Preparation, Characterization and Antibacterial Activity
Shikha Katiyar1 , Devendra Pratap Rao1*
, Devendra Pratap Rao1* , Narendra Kumar Verma1
, Narendra Kumar Verma1 , Amit Kumar Gautam1
, Amit Kumar Gautam1 , Ashish Verma1
, Ashish Verma1 , Chandra Prakash Singh2
, Chandra Prakash Singh2 , Vijay Shankar3
, Vijay Shankar3 Yashveer Gautam4
Yashveer Gautam4
1Department of Chemistry, Coordination Chemistry Laboratory, Dayanand Anglo-Vedic (PG) College, Kanpur-208001, U.P., India.
2Department of Chemistry, Mahatma Gandhi (PG) College, Gorakhpur- 273001, U.P., India.
3Department of Chemistry, B.S.N.V. (PG) College, Lucknow-226001, U.P., India.
4Department of Chemistry, Pandit Prithi Nath (PG) College, Kanpur-208001, U.P., India.
Corresponding Author E-mail: devendraprataprao@yahoo.com
DOI : http://dx.doi.org/10.13005/ojc/400104
Article Received on : 09 Dec 2023
Article Accepted on : 29 Jan 2024
Article Published : 13 Feb 2024
Reviewed by: Dr. Ahmed A. J. Mahmood
Second Review by: Dr. Deepak Yadav
Final Approval by: Dr. MGH Zaidi
Using di-2-furanylethanedione and 5-bromo-3-methylbenzene-1,2-diamine we prepared a monomeric [MoO2(SL)] with a Schiff base, as well as 4 different compounds using the formulation [MoO2(MSL). We investigate how [MoO2(SL)] reacts with 1,3-diketones. Several characterizations are discussed in this article, including molar conductance measurement, elemental analysis, UV-Vis, IR, NMR, and thermal measurements. Molybdenum has a six-coordination number. All five MoO2(VI) compounds have distorted octahedral arrangements. Molybdenum octahedra have four N-atoms and two oxidized O-atoms. Against S. aureus and S. typhi, all synthesized compounds showed moderate activity. The chelation hypothesis is used to define the progression of the antibacterial task.
KEYWORDS:Antibacterial Activity; β-diketones; 5-bromo-3-methylbenzene-1,2-diamine; Dioxomolybdenum(VI); di-2-furanylethanedione; Macrocyclic Compounds; Schiff base
Download this article as:| Copy the following to cite this article: Katiyar S, Rao D. P, Verma N. K, Gautam A. K, Verma A, Singh C. P, Shankar V, Gautam Y. Dioxomolybdenum (VI) Compounds of Macrocyclic Schiff base Ligands: Preparation, Characterization and Antibacterial Activity. Orient J Chem 2024;40(1). |
| Copy the following to cite this URL: Katiyar S, Rao D. P, Verma N. K, Gautam A. K, Verma A, Singh C. P, Shankar V, Gautam Y. Dioxomolybdenum (VI) Compounds of Macrocyclic Schiff base Ligands: Preparation, Characterization and Antibacterial Activity. Orient J Chem 2024;40(1). Available from: https://bit.ly/3wcSScp |
Introduction
At least nine atoms are required for a macrocyclic compound, including all heteroatoms. This type of compound contains at least three donor atoms. Over the past half century, macrocyclic compounds have undergone considerable development 1-5. In coordination chemistry, N-atom-containing ligands and their compounds play an important role. There have been various research papers published that explore metal compounds from physicochemical and biochemical perspectives and drive extensive applications 6-11. A transitional Schiff base formulation with vitamin B6 catalyzes transamination reactions using metal ions 15,16. In addition to their antifungal and antiviral properties, Schiff base compounds are also anti-inflammatory and antitumor 12-20.
Metalloproteins and enzymes contain positions of metal that can be modelled by Schiff base compounds in the bioinorganic field 21. In comparison to the isolated ligand, the anticancer activity of various Schiff bases is greater for that particular metal compound 22-29. Additionally, they remain fascinated by chemistry reactions and consumer electronics memory storage gadgets.
Extreme density ligands in transition metal compounds have different configurations and coordination numbers 30,31. The multiple oxidation states of molybdenum can allow it to be adaptable in this aspect. A coordination number may also range from 4 to 8 32. Schiff base edifices can be produced for homogeneous and heterogeneous reactions by linking molybdenum compounds with ligands containing hetero atoms (N, O and S) 33-39. There are a number of biological applications that can be achieved by MoO2(VI) compounds because they have multidentate ligands. [MoO4]2- can readily be acquired in aqueous solution as Mo(VI). Solution concentration and pH can determine the concentration of the [MoO4]2- ion. A template for oxygen transfer has been shown to exist in the form of the [MoO4]2- ion. It has been shown that their oxygen transfer properties have a negative effect on the molybdenum oxotransferase mechanism 40-43. It is known that several redox enzymes are fully oxidized. A cis-dioxomolybdenum moiety is associated with their active sites in such cases44-46.
Molybdenum is the only transition metal considered as a biometal that is essential for pathogenic microorganisms found in human, animal and vegetation pathogens 47,48. The biological and catalytic properties of Mo(VI) coordinate chemistry make it an active area of scientific research 49-52. A molybdenum molecule sets up the physiological functions of oxomolybdoenzymes 53-55. It might be useful as a chelating agent since di-2-furanylethanedione is versatile. There could be Schiff base condensation between diamines and di-2-furanylethanedione due to its reactive carbonyl groups. In order to synthesize macrocyclic ligands, di-2-furanylenedione plays an essential role.
Di-2-furanylethanedione has denticity ligands, which can be synthesized from reaction of diamine with di-2-furanylethanedione under certain conditions. Through metal template impact, the synthesized compound undergoes cyclization with 1,3-diketones. It is possible to prepare and characterize them, and TGA, nmr, ir, UV-Vis spectroscopy, molar conductivity measurements, and elemental analysis have all supported their provisional structures.
Experimental Section
Materials
Chemists obtained from business resources prepared Schiff bases (ligands) and compounds using reagent grade chemicals. In addition to molybdenyl acetylacetonate, 5-bromo-3-methylbenzene-1,2-diamine, and di-2-furanylethanedione, 1,3-diketones were obtained from Aldrich without similar refinement.
Physical Measurements and Analytical Methods
In Arunachal Pradesh, India, CHN analysers were used at important CRFs, including NERIST, Nirjuli, Itanagar, to examine C, H and N in the compounds. Nitrogen is assessed using Kjeldahl’s method for synthesized compounds. Using a gravimetric technique, molybdenum was measured after decay of the compound 56. As barium sulfate was developed as a method for estimating sulfur, sulfur estimation progressed 57. In addition to sulfuric acid baths, general methods were used for determining uncorrected melting factors. A Labinda-UV 3000+ UV/VIS spectrophotometer at UPTTI Kanpur, U.P., India was used to compute the electronic absorption spectra of the compounds utilizing ethanol. At Indian Institute of Technology Kanpur, we recorded the infrared spectra of MoO2(VI) compounds in KBr using Perkin-Elmer Spectrum models 10.03.06 and 18.03.06 spectrophotometers. On the JMM ECS-400 (JEOL) spectrometer, proton (1H NMR) spectra of MoO2(VI) compounds were obtained. An analysis of the temperature distribution of the [MoO2(SL)] compound was carried out using the TG/DTA-SDT Q600 V 20.9 Built 20, USA thermal analyzer under a nitrogen atmosphere at temperatures between 50 – 900 °C at a10 °C min-1 heating rate.
Preparation of molybdenum(VI) compounds containing ligands that result from condensation of di-2-furanylethanedione and 5-bromo-3-methylbenzene-1,2-diamine with 1,3-diketones
Schemes 1, 2 and 3 show how to synthesize [MoO2(MSL)]. Drop-wise addition of molybdenyl acetylacetonate (1.6307 g, 5 mmol) in ethanol (50 mL) was followed by the addition of di-2-furanylethanedione (0.9506 g, 5 mmol) in 50 mL ethanol and 5-bromo-3-methylbenzene-1,2-diamine (2.0106 g, 10 mmol) in 50 mL ethanol. The solution turns brown after 3 hours of slight reflux in the reaction mixture. After filtering, ethanol was used to wash the solid product, and silica gel was used to isolate the solid under vacuum. In order to check the purity of the compound, the TLC method was used. As a result, the yield increased to 48% (type A). 3D structures of the Schiff base ligand (SL) and parent compound [MoO2(SL)] are given in figure 1 and 2.
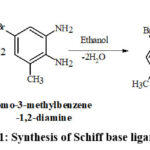 |
Scheme 1: Synthesis of Schiff base ligand (SL) |
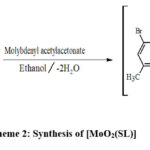 |
Scheme 2: Synthesis of [MoO2(SL)] |
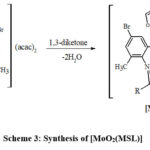 |
Scheme 3: Synthesis of [MoO2(MSL)] |
Where, SL = di-2-furanylethanedione + 5-bromo-3-methylbenzene-1,2-diamine; MSL = in the presence of dioxmolybdenum(VI) cation, macrocyclic ligands are synthesized by condensation of SL with 1,3-diketones.
MSL1: R = CH3, R’ = CH3; MSL2: R = C6H5, R’ = CH3; MSL3: R = C4H3S, R’ = CF3; MSL4: R = C6H5,R’ = C6H5
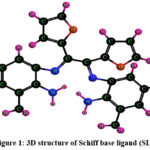 |
Figure 1: 3D structure of Schiff base ligand (SL) |
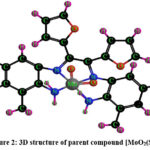 |
Figure 2: 3D structure of parent compound [MoO2(SL)] |
Upon addition of 1,3-diketones to ethyl alcohol suspension of Kind A, a uniform reaction was observed after three hours. Here we used 1,3-diketones like 4,4,4-trifluoro-1-(2-thienyl)-1,3-butanedione, 1,3-diphenyl-1,3-propanedione, 2,4-pentanedione or 1-phenyl-1,3-butanedione (1:1) to produce yellow solid macrocyclic stable products (type B). A TLC test is performed to verify the purity of macrocyclic compounds.
Table 1: Schiff base ligands and compounds: physical and analytical data.
|
Compound |
Formula |
F.W. |
Yield (%) |
m.p. (0C) |
% of Element, Calcd. / (found) |
||||
|
C |
H |
N |
Mo |
S |
|||||
|
SL |
C24H20Br2N4O2 |
556.26 |
65 |
120 |
51.82 (51.74) |
3.62 (3.60) |
10.07 (10.00) |
— |
— |
|
[MoO2(SL)] |
C34H34Br2N4 MoO8 |
882.41 |
55 |
118 |
46.27 (46.20) |
3.88 (3.78) |
6.34 (6.38) |
10.87 (10.80) |
— |
|
[MoO2(MSL1)] |
C39H38Br2N4 MoO8 |
946.52 |
50
|
130
|
49.49 (49.40) |
4.05 (4.00) |
5.92 (6.86) |
10.14 (10.9) |
— |
|
[MoO2(MSL2)] |
C44H40Br2N4 MoO8 |
1008.59 |
45
|
155
|
52.40 (52.33) |
4.00 (3.96) |
5.56 (5.32) |
9.51 (9.46) |
— |
|
[MoO2(MSL3)] |
C42H38Br2N4 MoO8F3S |
1071.59 |
45
|
125
|
47.05 (46.90) |
3.57 (3.50) |
5.22 (5.15) |
8.95 (8.89) |
2.99 (2.95) |
|
[MoO2(MSL4)] |
C49H42Br2N4 MoO8 |
1070.66 |
50
|
132
|
54.97 (54.94) |
3.95 (3.91) |
5.23 (5.20) |
8.96 (8.90 |
— |
Where,
SL= Di-2-furanylethanedione has been transformed into a Schiff base by condensation with 5-bromo-3-methylbenzene-1,2-diamine; Ligand MSL1 synthesized via condensation of SL with 2,4-pentanedione to form a macrocyclic Schiff base; Ligand MSL2 synthesized via condensation of SL with 1-phenyl-1,3-butanedione to form a macrocyclic Schiff base; Ligand MSL3 synthesized via condensation of SL with 4,4,4-trifluoro-1-(2-thienyl)-1,3-butanedione to form a macrocyclic Schiff base; Ligand MSL4 synthesized via condensation of SL with 1,3-diphenyl-1,3-propanedione to form a macrocyclic Schiff base.
According to elemental analysis (table 1), metal and ligand are in a 1:1 stoichiometry.
Antibacterial activity assay
Several bacterial strains were tested in vitro for their antibacterial activity, including S. aureus, B. subtilis, E. aerogene and S. typhi utilizing an agar-well diffusion process [58-60]. Antibacterial activity of Doxycycline became the same as that of other antibiotics. Agar media were drilled with 6 mm-diameter holes using a metallic borer. All bacterial suspensions were adjusted to a density of 3×105 colony-forming units per mL. Agar was expanded with standardized suspensions. In the examined sample, 300 g mL-1 of the produced compounds were dissolved in 1% DMSO. Each well was filled with the examined samples. DMSO and Doxycycline (0.05%) were packed in the rest of the wells. Upon incubation at 355 °C for 30 hours, growth inhibition is tested.
Results and Discussion
Infrared spectra
Through an in-situ procedure, dioxomolybdeum(VI) was produced by refluxing a mixture of di-2-furanylethanedione, 5-bromo-3-methylbenzene-1,2-diamine, and molybdenyl acetylacetonate in aqueous ethanol in a 1:2:1 ratio, which resulted in macrocyclic compounds as shown in the scheme. Several infrared bands were observed for the isolated ligands and the MoO2(VI) compounds. Table 2 highlights these bands. A macrocyclic compound with N-atoms of the group azomethine bonded to molybdenum has been demonstrated by lowering νC=N absorption frequencies 61-64. In isolated ligands, >C=N absorption appears at 1665 cm-1 in spectrum bands between 1610-1650 cm-1 61-63. It could be assigned to the Mo-N vibration around 490-570 cm-1, which is not related to free ligands 65. Di-2-furanylethanedione forms a strong bond with diamines due to the presence of two >C=N bands around 1710 cm-1 66,67. Dioxomolybdeum(VI) compounds and their isolated ligands exhibit compound infrared spectra due to various ring vibrations and C-H vibrations. An asymmetric (N-H) and symmetric (N-H) wide band is targeted at 3430 and 3060 cm-1, respectively. There is no difference between [MoO2(SL)] and [MoO2(MSL)] because the NH group is absent. This implies the NH group is not involved in the bonding process 68. Among dioxomolybdenum(VI) compounds, d-orbital is used most often for bonding, forming preferentially cis-dioxo groups. In dioxomolybdenum(VI) compounds, cis-[MoO2]2+ demonstrates asymmetric and symmetric stretching vibrations at 896-910 cm-1 and 962-970 cm-1 as a result of C2V symmetry 69. There are two infrared spectral bands at these wavelengths that are associated with νasym(O=Mo=O) and νsym(O=Mo=O) vibrations respectively89-76. In comparison to νsym(O=Mo=O), νasym(O=Mo=O) vibrations are lower 77,78. On the outer coordination sphere, the acetylacetonate group can be observed in the bands around 1555-1570 cm-1 and 1465-1482 cm-1, which correspond to the νC=O and νC=C vibrations, respectively [79]. The macrocyclic compounds exhibit an equal pattern of infrared spectral bands. A method of cyclizing 1,3-diketones by containing amino groups on the carbonyl group, the asymmetrically and symmetrically stretching vibrations of terminal amino groups disappear 79,80.
Table 2: Schiff base ligand and dioxomolybdenum compounds IR spectral bands (ν / cm-1). In the range of 4000 – 400 cm-1, all spectra were recorded with KBr.
|
Compound |
ν of |
|||||||
|
C=N |
Mo-N |
C=O (acac) |
C=C (acac) |
asym (O=Mo=O) |
sym (O=Mo=O) |
asym (N-H) |
ym(N-H)
|
|
|
SL |
1660m |
— |
— |
— |
— |
— |
3325br |
3133br |
|
[MoO2(SL) |
1610s |
490m |
1570m |
1465s |
901s |
965s |
3430br |
3060br |
|
[MoO2(MSL1)] |
1642m |
555s |
1565s |
1480m |
905m |
965s |
|
|
|
[MoO2(MSL2)] |
1650s |
562s |
1560s |
1472s |
910m |
970m |
|
|
|
[MoO2(MSL3)] |
1630s |
570m |
1555m |
1470m |
904m |
972m |
|
|
|
[MoO2(MSL4)] |
1645m |
530s |
1563s |
1470s |
896s |
965s |
|
|
1H NMR spectra
All NMR signals are given in table 3. All MoO2(VI) compounds as well as the ligand were analyzed using 1H NMR in DMSO-D6. It is possible that SL contains signal because of NH2 at δ (5.32), which is also found in [MoO2(SL)], at δ (7.20) but not in other macrocyclic compounds, which suggests the presence 1,3-diketones that are present in cyclization [84-86]. For isolated ligands and molybdenum compounds, the ten protons are multiplets within the range δ (7.84-6.53). About δ 7.25 peaks appeared for the protons of aromatic rings. In the preparation of the macrocyclic compound, two types of azomethine are offered which appear to result in these chemical shifts. It is possible that peak at δ 2.33 is be due to CH3 attached to an aryl group.
Table 3: Dioxomolybdenum compounds and ligand 1HNMR Spectral Data (in δ ppm).
|
Compound |
HC-Ar |
N-H |
H3C-Ar |
=C-CH3 |
-CH2 |
HC-furan |
HC- thienyl |
|
SL |
7.12 2H 7.17 2H |
5.32 4H |
|
2.12 6H |
— |
6.54 4H 7.84 2H |
— |
|
[MoO2(SL)] |
7.32 2H 7.40 2H |
7.20 4H |
|
2.33 6H |
— |
6.54 4H 7.84 2H |
— |
|
[MoO2(MSL1)] |
7.32 2H 7.40 2H |
— |
2.33 6H |
0.87 6H |
1.05 2H |
6.54 4H 7.84 2H |
— |
|
[MoO2(MSL2)] |
7.32 4H 7.40 5H |
— |
2.33 6H |
0.87 3H |
1.05 2H |
6.54 4H 7.84 2H |
— |
|
[MoO2(MSL3)] |
7.32 2H 7.40 2H |
— |
2.33 6H |
0.87 3H |
1.05 2H |
6.54 4H 7.84 2H |
7.01 1H 7.15 1H 7.53 1H |
|
[MoO2(MSL4)] |
7.32 6H 7.40 8H |
— |
2.33 6H |
— |
1.05 2H |
6.54 4H 7.84 2H |
— |
UV – Visible spectra
These spectra were measured in ethanol, and they are consistent with the strength energy level scheme suggested by the tetradentate tetraaza ligand and dixomolybdenum(VI) compounds [81-83]. Tetradentate ligands and dixomolybdenum(VI) compounds exhibit similar spectra. With the Mo(VI) ion having no d- electron, it is no longer expected to show pure d-d absorption bands. All molybdenum compounds can also be explained as charge transfer transitions between their nitrogen and molybdenum d-orbitals [N(π)→d(Mo)]. A dioxomolybdenum(VI) compound containing nitrogen donor atoms exhibits homogeneous UV-VIS spectra. Strong absorption bands during the UV – Vis spectrum of those compounds is identified at 292 nm and at 311 nm, which may be attributed to intraligand transitions and n→π* / π→π* transitions. The band between 380 nm and 395 nm appears to be caused by N(π)→d(Mo). Transition 2B2→2A1 (dxy→dx2-y2) may be covered within other bands and is ideal to facilitate charge transfer between the molybdenum LUMO and the ligand HOMO. Energy level schemes for these compounds have been provided by Ballhausen-Gray diagrams. Each compound’s electronic spectrum has an octahedral distortion [87].
Magnetic and molar conductance measurements
As for d0 configuration, it is not necessary to mention that dioxomolybdenum(VI) compounds are diamagnetic. Due to the absence of electrons in d-orbitals, no d-d transitions are determined for those compounds. The molar conductivities (ΛM) of all molybdenum compounds in DMF at the ca. 10-3 M endorse 1:1 sort electrolytes. Those compounds have molar conductance ranging from 90 to 110 Ω-1 cm2 mol-1. As predicted by the schemes, the molar conductance values on top of the compounds of dioxomolybdenum(VI) type (A) and macrocyclic type (B) suggest tentative shapes.
Thermogravimetric analyses
Thermogravimetric curve (TG Curve) of the compound presented in figure 3 reveals that the compound has decomposed in one step. At a temperature of 279 ℃ about 83% weight loss has been observed which is due to loss of Schiff ligand and acetylacetonate moiety (calculated weight 83.1%). At 281 ℃, the residue left is 16.3% which corresponds to MoO3. Weight percent left at 324 ℃ is 14.48 and is attributed to MoO2.
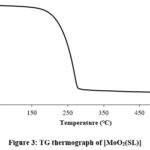 |
Figure 3: TG thermograph of [MoO2(SL)] |
Antibacterial activity
As can be seen in table 4, the dioxomolybdenum(VI) compounds exhibit antimicrobial activity. It was tried against a variety of bacteria, including S. aureus, B. subtilis, E. aerogenes, and S. typhi, using the synthesized dioxomolybdenum(VI) compounds.Chelation hypothesis can be used to explain the improvement of dioxomolybdenum(VI) compounds’ antibacterial properties [79,80]. A doxycycline remedy is used as the reference material. Many of the compounds were found to have low or modest activity against S. aureus and S. typhi.
Using Eq 1 as a starting point, we calculate the percentage of inhibition effect based on the positive control.
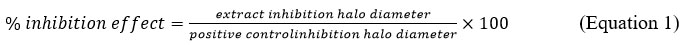
Table 4: Antibacterial activitiesa
|
Compound |
HC-Ar |
N-H |
H3C-Ar |
=C-CH3 |
-CH2 |
HC-furan |
HC- thienyl |
|
SL |
7.12 2H 7.17 2H |
5.32 4H |
|
2.12 6H |
— |
6.54 4H 7.84 2H |
— |
|
[MoO2(SL)] |
7.32 2H 7.40 2H |
7.20 4H |
|
2.33 6H |
— |
6.54 4H 7.84 2H |
— |
|
[MoO2(MSL1)] |
7.32 2H 7.40 2H |
— |
2.33 6H |
0.87 6H |
1.05 2H |
6.54 4H 7.84 2H |
— |
|
[MoO2(MSL2)] |
7.32 4H 7.40 5H |
— |
2.33 6H |
0.87 3H |
1.05 2H |
6.54 4H 7.84 2H |
— |
|
[MoO2(MSL3)] |
7.32 2H 7.40 2H |
— |
2.33 6H |
0.87 3H |
1.05 2H |
6.54 4H 7.84 2H |
7.01 1H 7.15 1H 7.53 1H |
|
[MoO2(MSL4)] |
7.32 6H 7.40 8H |
— |
2.33 6H |
— |
1.05 2H |
6.54 4H 7.84 2H |
— |
aIn vitro diffusion method using cups and wellsconc. 300 μg mL-1 in solvent DMSO; Inhibition zone (mm): 23-26 = strong activity; 15-18 = moderate activity; 19-22 = good activity; an antibiotic drug of reference is doxycycline.
InChl
InChI=1S/C24H20Br2N4O2/c1-13-9-15(25)11-17(21(13)27)29-23(19-5-3-7-31-19)24(20-6-4-8-32-20)30-18-12-16(26)10-14(2)22(18)28/h3-12H,27-28H2,1-2H3/b29-23+,30-24+
InChl key of compound
VWNWPFYNPOEBGW-HCTXVGCHSA-N
SMILES Notation
Brc4cc(\N=C(\C(=N\c1cc(Br)cc(C)c1N)\c2ccco2)c3ccco3)c(N)c(C)c4
LogP
5.50+/- 0.67
Conclusion
These compounds haven’t been crystallized yet, so their crystal structures are unavailable. The [MoO2(SL)] and [MoO2(MSL)] compounds can be represented using the above elemental and spectral studies. The antibacterial activity of these compounds has been tested. We demonstrate the formation of dioxin derivatives from dioxomolybdenum(VI) Schiff bases. The Schiff base condensation was confirmed using a flexible chelator with two carbonyl groups, di-2-furanylethanedione. As a result of their reaction with 1,3-diketones, macrocyclic products are formed. Distorted octahedron surrounding Mo. Di-2-furanyledione and diamines are used to condense Schiff bases in ethanol medium. Tetradentate bonds are formed between azomethine nitrogen atoms and metal ions in Schiff bases. One metal ion is present in each ligand molecule. Compounds prepared have been shown to have six coordinated distorted octahedral shapes and a mononuclear six coordination.
Acknowledgment
It would be appreciated if the authors could thank the BoM-Secretary, D.A-V. Post-Graduate College, Kapur for the opportunity to look at the work in a laboratory setting. Fortuitously, UPTTI Kanpur India supplies analytical facilities.
Conflict of Interest
Financial interests of the authors are not in conflict.
Funding Sources
As part of this work, we received support from the Directorate of Higher Education, Uttar Pradesh, Prayagraj (Degree Vikas/225-232/2021-2022 dated 01 April 2021).
References
- Healy, M.D.S.; Rest, A.J. Template reactions. Advances Inorganic Chemistry and Radiochemistry1978, 21, 1-40, https://doi.org/10.1016/S0065-2792(08)60277-0
CrossRef - Sergienko, V.S.; Abramenko, V.L.; Gorbunova, Y.E. Dioxomolybdenum (VI) Complexes with R1-Substituted Salicylidene Allylimines (Hl n): Synthesis and Structure. Crystal Structure of [MoO 2 (L 1) 2](R 1= H). Russian Journal of Inorganic Chemistry 2018, 63, 28-33, https://doi.org/10.1134/S003602361801014X
CrossRef - Melson, G.A. Coordination Chemistry of Macrocyclic Compounds. Plenum Press, NY, 1979.
CrossRef - Yadava, A.K.; Yadav, H.S.; Rao, D.P. Syntheses and spectral characterization of some novel macrocyclic complexes of oxovanadium(iv) with 1,1’-oxalyldiimidazole. European Chemical Bulletin2013, 2, 255-258. https://doi.org/10.17628/ECB.2013.2.255
CrossRef - Lindoy, L.F.; Busch, D.H. Metal ion-controlled syntheses of novel five-coordinate zinc and cadmium complexes containing a helical coordination geometry and their template reaction to form complexes of a pentadentate macrocyclic ligand. Inorganic Chemistry1974, 13(10), 2494-2498, https://doi.org/10.1021/ic50140a037
CrossRef - Luo, X.F.; Hu, X.; Zhao, X.Y.; Goh, S.H.; Li, X.D. Miscibility and interactions in blends and complexes of poly(4-methyl-5-vinylthiazole) with proton-donating polymers, Polymer2003, 44(18),5285-5291, https://doi.org/10.1016/S0032-3861(03)00578-0
CrossRef - Murthy, A.S.N.; Reddy, A.R. Electronic absorption spectroscopic studies of enolimine-ketoamine equilibria in Schiff bases. Journal of Chemical Scences1981, 90(6), 519-526, https://www.ias.ac.in/article/fulltext/jcsc/090/06/0519-0526
CrossRef - Razakantoanina, V.; Phung, N.K.P.; Jaureguiberry, G. Antimalarial activity of new gossypol derivatives, Parasitology Research2000, 86(8),665-668, https://doi.org/10.1007/pl00008549
CrossRef - Royer, R.E.; Deck, L.M.; Vander T.J.; Jagtm, Synthesis and anti-HIV activity of 1,1′-dideoxygossypol and related compounds, Journal of Medicinal Chemistry1995, 38(13), 2427-2432, https://doi.org/10.1021/jm00013a018
CrossRef - Flack, M.R.; Pyle, R. G.; Mullen, N.M. Oral gossypol in the treatment of metastatic adrenal cancer. Journal of Clinical Endocrinolology Metabolism1993, 76 1019-1024, https://doi.org/10.1210/jcem.76.4.8473376
- Baumgrass, R.; Weiwad, M.; Erdmann, F. Reversible inhibition of calcineurin by the polyphenolic aldehyde, gossypol, The Journal of Biological Chemistry2001, 276(51),47914-47921, https://doi.org/10.1074/jbc.m103273200
CrossRef - Al-Shihri, A.S.M.; Abdel Fattah, H.M. Thermogravimetric and Spectroscopic characterization of trivalent lanthanide chelates with some Schiff base. Journal of Thermal Analysis Calorimetry2003, 71(2), 643-649, https://doi.org/10.1023/A:1022880615841
CrossRef - Raman, N.; Sakthivel, A.; Rajasekaran K. Synthesis and spectral characterization of antifungal sensitive Schiff base transition metal complexes. Mycobiology2007, 35(3), 150-153, https://doi.org/10.4489/MYCO.2007.35.3.150
CrossRef - Creaven, B.S.; Czeglédi, E.; Devereux, M.; Enyedy, E.A.; Kia, A.F.A; Karcz, D.; Kellett, A.; McClean, S.; Nagy, N.V.; Noble, A.; Rockenbauer, A.; Szabó-Plánka, T.; Walshab, M. Biological activity and coordination modes of copper(II) complexes of Schiff base-derived coumarin ligands. Dalton Transactions2010, 39, 10854-10865, https://doi.org/10.1039/c0dt00068j
CrossRef - Doctor, V.M.; Oro, J. Mechanism of non-enzymatic transition reaction between histidine and alphaoxoyglutaric acid. Journal of Biochemistry1969, 112(5),691-697, https://doi.org/10.1042/bj1120691
CrossRef - El-Gammal, O.A.; El-Reash, G.M.A.; Goama, H.E. Mononuclear Cr(III), Mn (II), and Fe(III) complexes derived from new ONO symmetrical flexible hydrazone: synthesis, spectral characterization, optical band gap and DFT computational study. Letters in Applied NanoBioScience2019, 8(4), 743-753, https://doi.org/10.33263/LIANBS84.743753
CrossRef - Jarrahpour, A.; Khalili, D.; De Clercq, E.; Salmi, C.; Brunel, J.M. Synthesis, antibacterial, antifungal and antiviral activity evaluation of some new bis-Schiff bases of isatin and their derivatives. Molecules2007, 12, 1720-1730, https://doi.org/10.3390/12081720
CrossRef - Bharti, S.K.; Patel, S.K.; Nath, G.; Tilak, R.; Singh, S.K. Synthesis, characterization, DNA cleavage and in vitro antimicrobial activities of copper(II) complexes of Schiff bases containing a 2,4-disubstituted thiazole. Transition Metal Chemistry2010, 35, 917-925, https://doi.org/10.1007/s11243-010-9412-8
CrossRef - Manjunatha, M.; Naik, V. H.; Kulkarni, A.D.; Patil, S.A. DNA cleavage, antimicrobial, antiinflammatory anthelmintic activities, and spectroscopic studies of Co(II), Ni(II), and Cu(II) complexes of biologically potential coumarin Schiff bases. Journal of Coordination Chemistry2011, 64(24), 4264-4275, https://doi.org/10.1080/00958972.2011.621082
CrossRef - Amer, S.; El-Wakiel, N.; El-Ghamry, H. Synthesis, spectral, antitumor and antimicrobial studies on Cu(II) complexes of purine and triazole Schiff base derivatives. Journal of Molecular Structure2013, 1049, 326-335, https://doi.org/10.1016/j.molstruc.2013.06.059
CrossRef - Ohashi, M.; Koshiyama, T.; Veno, T.; Yanase, M.; Fujii, H.; Watanabe, Y. Preparation of artificial metalloenzymes by insertion of Chromium(III)Schiff base complexes in to apomyoglobin mutants. Angewandte Chemie International Edition2003, 42(9), 1005-1008, https://doi.org/10.1002/anie.200390256
CrossRef - Dongfang, X.U.; Shuzhi, M.A.; Guangyinng, D.V.; Qizhuang, H.E.; Dazhi, S. Synthesis, characterization and anticancer properties of rare earth complexes with Schiff base and O-phenanthroline. Journal of Rare Earths2008, 26(5), 643-647, https://doi.org/10.1016/S1002-0721(08)60153-2
CrossRef - Uddin, N.; Rashid, F.; Ali S.; Tirmizi S.A.; Ahmad, I.; Zaib, S.;Zubair, M.; Diaconescu, P.L.; Tahir, M.N., Jamshed, I.; Haider, A. Synthesis, characterization, and anticancer activity of Schiff bases. Journal of Biomolecular Structure and Dynamics2019, 38(11), 3246-3259, https://doi.org/10.1080/07391102.2019.1654924
CrossRef - Etain, S.E.H.; Abd El-Aziz, D.M.; Abd El-Zaher, E.H.; Ali, E.A. Synthesis spectral, antimicrobial and antitumor assessment of Schiff base derived from 2-aminobenzothiazol and its transition metal complexes. Spectro Chim.Acta. Molecular and Biomolecular Spectroscopy, A2011. 79(5),1331-1337, https://doi.org/10.1016/j.saa.2011.04.064
CrossRef - Cozzi, P.G. Metal-Salen Schiff base complexes in catalysis: practicalaspects. Chemical Society Reviews2004; 33, 410–421, https://doi.org/10.1039/b307853c
CrossRef - Singh, R.K.; Kukrety, A.; Saxena, R.C.; Thakre, G.D.; Atray, N.; Ray, S.S. Novel triazine Schiff base-based cationic gemini surfactants: Synthesis and their evaluation as Antiwear, antifriction, and anticorrosive additives in polyol. Industrial and Engineering Chemistry Research2016, 55(9), 2520-2526, https://doi.org/10.1021/acs.iecr.5b04242
CrossRef - Sathya, N.; Raja, G.; Padma; Priya, N.; Jayabalakrishnan C. Ruthenium(II) complexes incorporating tridentate schiff base ligands: Synthesis, spectroscopic, redox, catalytic and biological properties. Applied Organometallic Chemistry2010, 24(5), 366-373, https://doi.org/10.1002/aoc.1621
CrossRef - Arun, V.; Sridevi, N.; Robinson, P.P.; Manju, S.; Yusuff, K.K.M. Ni(II) and Ru(II) Schiff base complexes as catalysts for the reduction of benzene. Journal of Molecular Catalysis A: Chemical2009, 304(1-2), 191-198, https://doi.org/10.1016/j.molcata.2009.02.011
CrossRef - Segura, J.L.; Mancheño, M.J.; Zamora, F. Covalent organic frameworks based on Schiff-base chemistry: synthesis, properties and potential applications. Chemical Society Review2016, 45, 5635-5671, https://doi.org/10.1039/c5cs00878f
CrossRef - Yadava, H.D.S.; Sengupta, S.K; Tripathi, S.C. Syntheses and spectroscopic studies on dioxouranium(VI), oxovanadium(IV) and oxozirconium(IV) complexes with tetradentate macrocyclic ligands. Inorganica Chimica Acta1987, 128, 1-6, https://doi.org/10.1016/S0020-1693(00)84685-X
CrossRef - Sergienko, V.S.; Abramenko, V.L.; Surazhskaya, M.D. Intracomplex Dioxomolybdenum (VI) Compounds with Alcoholimines of Aromatic o-Hydroxyaldehydes. Crystal Structure of 2-Hydroxynaphthylidene Monoethanolimine (H2 L) and Solvated Complex [MoO2(L)·C5H5N]. Russian Journal of Inorganic Chemistry 2020, 65, 495-501, https://doi.org/10.1134/S0036023620040166
CrossRef - Crans, D.C. Fifteen years of dancing with vanadium. Pure and Applied Chemistry2005. 77(9), 1407-1527, http://dx.doi.org/10.1351/ pac200577091497
CrossRef - Bagherzadeh, M.; Amini, M.; Parastar, H. Synthesis, X-ray structure and oxidation catalysis of a oxido–peroxido molybdenum(VI) complex with a tridentate Schiff base ligand. Inorganic Chemistry Communications2012, 20, 86-89, https://doi.org/10.1016/j.inoche. 2012.02.023
CrossRef - Moradi-Shoeili, Z.; Zare.; M.; Bagherzadeh, M.; Kubicki, M.; Boghaei, D.M. Preparation and structure of zinc complexes containing pincer ligands and their application for Knoevenagel condensation in water. Journal of Coordination Chemistry2015, 68(2), 548-559, https://doi.org/10.1080/00958972.2014.978308
CrossRef - Bagherzadeh, M.; Amini, M.; Parastar, H. Synthesis, X-ray structure and oxidation catalysis of a oxido–peroxido molybdenum(VI) complex with a tridentate Schiff base ligand. Inorganic Chemistry Communication2012, 20, 86-89, https://doi.org/10.1016/j.inoche. 2012.02.023
CrossRef - Aziz, A.A.A. Synthesis, spectroscopic characterization, thermal studies, catalytic epoxidation and biological activity of chromium and molybdenum hexacarbonyl bound to a novel N2O2 Schiff base. Journal of Molecular Structure2010, 979(1-3), 77-85, https://doi.org/10.1016/ j.molstruc. 2010.06.004
CrossRef - Chakravarthy, R.D.; Suresh, K.; Ramkumar, V.; Chand, D.K. New chiral molybdenum complex catalyzed sulfide oxidation with hydrogen peroxide. Inorganica Chimica Acta2011, 376(1), 57-63, https://doi.org/10.1016/j.ica.2011.05.033
CrossRef - Rayati, S.; Rafiee, N.; Wojtczak, A. cis-Dioxo-molybdenum(VI) Schiff base complexes: Synthesis, crystal structure and catalytic performance for homogeneous oxidation of olefins. Inorganica Chimica Acta2012, 386, 27-35, https://doi.org/10.1016/j.ica.2012.02.005
CrossRef - Rao, D.P. A review on versatile applications of novel Schiff bases and their metal complexes. Letters in Applied NanoBioScience2019, 8(4), 675-681. https://doi.org/10.33263/LIANBS84.675681
CrossRef - Pushie, M.J.; George, G.N. Spectroscopic studies of molybdenum and tungsten enzymes. Coordination Chemistry Review2011, 255(9-10), 1055-1084, https://doi.org/10.1016/j.ccr.2011.01.056
CrossRef - Gautam, R.K.; Singh, C.P.; Prasad S.P.; Saxena, R.; Rao, D.P. Synthesis and antibacterial activity of novel molybdenum complexes with macrocyclic Schiff base derived from furanylethanedione. Asian Journal of Chemistry2019, 31, 2607-2612, https://doi.org/10.14233/ ajchem.2019.22242
CrossRef - Rao, C.P.; Sreedhara, A.; Rao, P.V.; Verghese, M.B.; Rissanen, K.; Kolehmainen, E.; Lokanath, N.K.; Sridhar, M.A.; Prasad, J.S. Syntheses, structure, reactivity and species recognition studies of oxo-vanadium(V) and -molybdenum(VI) complexes. Journal of the Chemical Society,1998, 14, 2383-2394, https://doi.org/10.1039/A801226A
CrossRef - Hahn, R.; Herrmann, W.A.; Artens, G.R.J. Kleine, M. Biologically relevant metal coordination compounds: MoVIO2 and nickel(II) complexes with tridentate aromatic Schiff bases. Polyhedron1995, 14(20-21), 2953-2960, https://doi.org/10.1016/0277-5387(95)00133-D
CrossRef - Mendel, R.R. Molybdenum: biological activity and metabolism. Dalton Transaction2005, 21, 3404-3409, https://doi.org/10.1039/B505527J
CrossRef - Sigel, A.; Sigel, H. Metal Ions in Biological Systems, Molybdenum and Tungsten: Their Roles in Biological Processes, Marcel Dekker, NY. 39, 2002.
- Maurya, R.C.; Shukla, B.; Pandey, A. Synthesis, magnetic and spectral studies of some cis-dioxomolybdenum(VI) complexes derived from N, O- and N2O2- type Schiff bases.Indian Journal of Chemistry2002, 41A(3), 554-559,URI: http://hdl.handle.net/123456789/29016
- Rousso, I,; Friedman, N.; Sheves, M.; Ottolenghi, M. pKa of the protonated Schiff base and aspartic 85 in the bacteriorhodopsin binding site is controlled by a specific geometry between the two residues. Biochemistry1995, 34, 12059-12065, https://doi.org/10.1021/bi00037a049
CrossRef - Bassov, T.; Sheves, M. Alteration of pKa of the bacteriorhodopsin protonated Schiff base. A study with model compounds. Biochemistry1980, 25(18), 5249-5258, https://doi.org/10.1021/ bi00366a040
CrossRef - Mimoum, H.; Roch, I.S.D.; Sajus, L. Epoxydation des olefines par les complexes peroxydiques covalents du molybdene-VI. Tetrahedron1970, 26(1), 37-50, https://doi.org/10.1016/0040-4020(70)85005-0
CrossRef - Conte, V.; Furia, F.D. Catalytic oxidations with hydrogen peroxide as oxidant, Kluwer Academic Publisher, Berlin, 1992.
- Mimoum, H.; Saussine, L.; Daire, E.; Postel, M.; Fisher, J.; Weiss, R. Vanadium(V) peroxy complexes. New versatile biomimetic reagents for epoxidation of olefins and hydroxylation of alkanes and aromatic hydrocarbons. Journal of American Chemistry Society1983, 105(10), 3101-3110, https://doi.org/10.1021/ja00348a025
CrossRef - Nair, M.L.H.; Thankamani, D. Synthesis and characterization of oxomolybdenum (V) and dioxomolybdenum (VI) complexes with schiff base derived from isonicotinoylhydrazide. Indian Journal of Chemistry2009, 48A(9), 1212-1218, URI: http://hdl.handle.net/123456789/6010
- Garner, G.D. Molybdenum, special topics in Comprehensive Coordination Chemistry. Wilkinson G, Ed., Pergamon press, Oxford. 1987, 6,1421.
- Niasari, M.S.; Davar, F.; Bazarganipour, M. Synthesis, characterization and catalyticoxidation of para-xylene by a manganese(III) Schiff base complex on functionalized multi-wall carbon nanotubes (MWNTs). Dalton Transactions2010, 39, 7330-7337, https://doi.org/10.1039/B923416K
CrossRef - Ambroziak, K.; Beleck, R.M.; He, Y.; Saha, B.; Sherrington, D.C. Investigation of batch alkene epoxidations catalyzed by polymer-supported Mo(VI) complexes. Industrial and Engineering Chemistry Research2009,48(7), 3293-3302, https://doi.org/10.1021/ie801171s
CrossRef - Vogel, A.I. A Text book of quantitative Inorganic analysis 4th ed., Longmans Green Co. Ltd., London, 1978.
- Vogel, A.I. A Text book of practical organic chemistry 4th ed., Longmans Green Co. Ltd., London, 1978.
- Simmons, A. Practical medical microbiology 14th ed, Churchill Livingston, Edinberg, 11, 163, 1996.
- Collee, J.G..; Duguid, J.P.; Frase, A.G.; Marmion, B.D. Practical medical microbiology, Churchill Livingstone, New York, 1989.
- Nag, P.; Sharma, D. Synthesis, characterization and anticandidal activity of dioxomolybdenum(VI) complexes of the type [MoO2{ON=C(CH3)Ar}2] and [MoO2{OC(R)CHC(R’)=NC6H5}2]. Heliyon2019, 5(5), E01729, https://doi.org/10.1016/j.heliyon.2019.e01729
CrossRef - Synthesis and structural characterization of novel square pyramidal oxovanadium(IV) complexes with ligands having N and O donor atoms. Turkish Journal of Chemistry2012, 36, 624-630, https://doi.org/10.3906/kim-1201-54
CrossRef - Chandra, S.; Sharma, K.K. Synthesis and characterization of copper(II) complexes of a macrocyclic ligand. Transition Metal Chemistry1983, 8, 1-3, https://doi.org/10.1007/BF00618784
CrossRef - Malik, W.U.; Bembi, R.; Singh, R. Preparation and characterisation of some new 12- and 14-membered dibenzotetraaza macrocyclic complexes of iron(III). Inorganica Chimica Acta1983,68, 223-228, https://doi.org/10.1016/S0020-1693(00)88965-3
CrossRef - Głowiak, T.; Jerzykiewicz, L.; Sobczak, J.M.; Ziółkowski, J.J. New insights into the chemistry of oxomolybdenum(VI) complexes with N-salicylidene-2-aminoethanol. Inorganica Chimica Acta2003,356, 387-392, https://doi.org/10.1016/S0020-1693(03)00301-3
CrossRef - Ferraro, J.R. Low frequency vibrations of inorganic and coordination compounds. Plenum, New York, 1971.
CrossRef - Dyer, J.R. Applications of absorption spectroscopy of organic compounds. Prentice-Hall, Inc., Englewood Cliffs, NJ, 1965.
CrossRef - Singh, S.; Yadav, H.S.; Yadava, A.K.; Rao, D.P. Synthesis and characterization of oxovanadium(IV) complexes with tetradentate Schiff base ligands having thenil as precursor molecule. Current Research in Chemistry2011, 3, 106-113, https://doi.org/10.3923/crc.2011.106.113
CrossRef - Willis, L.J.; Loehr, T.M.; Miller, K.F.; Bruce, A.E.; Stiefel, E.I. Raman and infrared spectroscopic studies of dioxomolybdenum(VI) complexes with cysteamine chelates. Inorganic Chemistry1986, 25,4289-4293, https://doi.org/10.1021/ic00243a045
CrossRef - Ceylan, B.I.; Kurt, Y.D.; Ulkuseven, B. Synthesis and characterization of new dioxomolybdenum(VI) complexes derived from benzophenone-thiosemicarbazone (H2L). Crystal structure of [MoO2L(PrOH)]. Journal of Coordination Chemistry2009, 62, 757-766, https://doi.org/10.1080/00958970802339669
- S.N. Rao, K.N. Munshi, N.N. Rao, M.M. Bhadbhade, E. Suresh. Synthesis, spectral and X-ray structural characterization of [cis-MoO2(L)(solv)](L= salicylidene salicyloyl hydrazine) and its use as catalytic oxidant. Polyhedron1999, 18, 2491-2497, https://doi.org/10.1016/S0277-5387(99)00139-4
CrossRef - El-Medani, S.M.; Aboaly, M.M.; Abdalla, H.H.; Ramadan, R.M. Reactions of Group 6 Metal Carbonyls with Salicylaldehyde Hydrazone. Spectroscopy Letter2004, 37(6), 619-632, https://doi.org/10.1081/SL-200037610
CrossRef - Zhu, X.W. Synthesis, crystal structures and catalytic property of dioxomolybdenum(VI) complexes derived from tridentate Schiff bases. Acta Chimica Slovenica2018, 65, 939-945, https://doi.org/10.17344/acsi.2018.4607
CrossRef - Maurya, R.C.; Chourasia, J.; Martin, M.H.; Roy, S.; Sharma, A.K.; Vishwakarma, P. Dioxomolybdenum(VI) chelates of bioinorganic, catalytic, and medicinal relevance: Studies on some cis-dioxomolybdenum(VI) complexes involving O, N-donor 4-oximino-2-pyrazoline-5-one derivatives. Arabian Journal of Chemistry2015, 8(3), 293-306, https://doi.org/10.1016/j.arabjc.2011.01.010
CrossRef - Wang, X.; Zhang, X.M.; Liu, H.X. Synthesis, characterization and crystal structure of cis-dioxomolybdenum(VI) complexes of the Schiff base Girard reagent (T) salt. Journal of Coordination Chemistry1994, 33, 223-228, https://doi.org/10.1080/00958979408024280
CrossRef - Mahmoudi, H.; Bagherzadeh, M.; Ataie, S.; Kia, R.; Heydar Moravej, S.; Zare, M.; Raithby, P.R.; Ferlin, F.; Vaccaro, L. Synthesis and X-ray crystal structure of a molybdenum(VI) Schiff base complex: Design of a new catalytic system for sustainable olefin epoxidation. Inorganica Chimica Acta2020, 511, 119775, https://doi.org/10.1016/j.ica.2020.119775
CrossRef - Rao, D.P.; Yadav, H.S.; Yadava, A.K.; Singh, S.; Yadav, U.S. Syntheses and spectroscopic studies on macrocyclic complexes of dioxomolybdenum(VI) with furil as precursor. E-Journal of Chemistry2012, 9, 497-503, https://doi.org/10.1155/2012/205123
CrossRef - Cotton, F.A.; Wilkinson, G.; Murillo, C.A.; Bochmann, M. Advanced Inorganic Chemistry. 6th ed., Wiley, New York, 1999, 18, 944-947.
CrossRef - Nakamoto, K. Infrared and Raman Spectra of inorganic and co-ordination compounds. New York: Wiley, 1978, https://doi.org/10.1002/0470027320.s4104
CrossRef - Gehrke, H.; Veal, J. Acetylacetonate complexes of molybdenum(V) and molybdenum(VI). Inorganica Chimica Acta1969,3, 623-627, https://doi.org/10.1016/S0020-1693(00)92563-5
CrossRef - Yadav, H.S. Synthesis of spectroscopic studies of oxovanadium(IV) complexes with 16- and 18-membered macrocyclic ligands. Polyhedron1993, 12, 313-317, https://doi.org/10.1016/S0277-5387(00)81729-5
CrossRef - Rao, D.P.; Yadav, H.S.; Yadava, A.K.; Singh, S.; Yadav, U.S. In-situ preparation of macrocyclic complexes of dioxomolybdenum(VI) involving a heterocyclic precursor. Journal of Coordination Chemistry2011, 64, 293-299,https://doi.org/10.1080/00958972. 2010.544037
- Rao, D.P.; Yadav, H.S.; Yadava, A.K.; Singh, S.; Yadav U.S. Synthesis and characterization of cis-dioxomolybdenum(VI) complexes having furil as precursor molecule. Journal of the Serbian Chemical Society,2012, 77, 1205-1210, https://doi.org/10.2298/JSC111110020
CrossRef - Gautam, R.K.; Singh, C.P.; Kumar, D.; Rao, D.P. New Insights into the Chemistry of cis-Dioxomolybdenum(VI) Schiff base complexes with macrocyclic ligands. Chemical Science Transactions2019, 8(4), 467-476, https://doi.org/10.7598/cst2019.1594
CrossRef - Garg, R.; Saini, M.K.; Fahmi, N.; Singh, R.V. Spectroscopic and biochemical studies of some manganese(II), oxovanadium(V) and dioxovanadium(VI) complexes S/O and N donor agents synthesized under microwave conditions. Transition Metal Chemistry2006, 31, 362-367, https://doi.org/10.1007/s11243-005-0001-1
CrossRef - Gautam, R.K.; Singh, C.P.; Saxena, R.; Rao, D.P. Synthesis and studies of some cis-MoO2(VI) complexes with nitrogen donor macrocyclic ligands. European Chemical Bulletin 2019, 8, 387-393, http://dx.doi.org/10.17628/ecb.2019.8.387-393
CrossRef - Kahroic, E.; Molcanov, K.; Tusek-Bozic, L.; Kojic-Prodic, B. New complexes of Mo(V) with Schiff bases: Crystal structure of butylammonium di-μ-oxo-μ-acetato-bis[(N-butylsalicylideniminato-N,O)oxomolybdenum(V)] benzene, acetic acid solvate. Polyhedron2006, 25(12), 2459-2464, https://doi.org/10.1016/ j.poly.2006.02.008
CrossRef - Ballhausen, C.J.; Gray, H.B. The electronic structure of the vanadyl ion. Inorganic Chemistry1962, 1, 111-122, https://doi.org/10.1021/ ic50001a022
CrossRef

This work is licensed under a Creative Commons Attribution 4.0 International License.









