Chiral Ionic Liquid-Based Vortex-Assisted Enantio-Separation of S-(+) and R-(-) Besifloxacin and Evaluation of Zero Point Energy by Two-Phase Liquid-Liquid Extraction
Eegala Bheema Shankar1 , Challa Gangu Naidu1,2
, Challa Gangu Naidu1,2 , Subramani Devaraju1*, K Varaprasada Rao4, Bondigalla Ramachandra3
, Subramani Devaraju1*, K Varaprasada Rao4, Bondigalla Ramachandra3 , Y. Srinivasa Rao4
, Y. Srinivasa Rao4 and Satwinder S Marok5
and Satwinder S Marok5
1Division of Chemistry, Department of Science and Humanities, Vignan's Foundation for Science, Technology and Research (Deemed to be University) VFSTRU, Vadlamudi, Guntur, Andhra Pradesh 522213, India.
2Department of Basic Sciences and Humanities (B S&H), Vignan’s Institute of Information Technology (VIIT), Besides VSEZ, Vadlapudi Duvvada, Visakhapatnam, Andhra Pradesh, 530049 India.
3Government College for Men, Andhra Pradesh, Kadapa, 516004 India.
4Department of Pharmaceutics, Vignan Institute of Pharmaceutical Technology (VIPT), Besides VSEZ, Vadlapudi Duvvada, Visakhapatnam, Andhra Pradesh, 530049 India.
5Apotex Inc, 150 Signet Drive, Toronto, ON, M9L1T9. Canada.
Corresponding Author E-mail: naiduiict@gmail.com
DOI : http://dx.doi.org/10.13005/ojc/400124
Article Received on : 18 Oct 2023
Article Accepted on : 16 Jan 2024
Article Published : 12 Feb 2024
Reviewed by: Dr.Ganesh Chaudhari
Second Review by: Dr. Satwashila kadam
Final Approval by: Dr. Malinee Sriariyanun
Besifloxacin is a fourth-generation fluoroquinolone and shows significant antibacterial properties, which works well against a range of bacteria. Besifloxacin ophthalmic solution is the name of the specific form of this medication used to treat eye infections. The vortex-assisted chiral ionic liquid method was used for the separation of Besifloxacin isomers. S- Besifloxacin shows antibacterial action in clinical trials. The R-isomer, however, has not shown biological properties in clinical testing through different cell line. The current proposed chiral assay method was developed between a racemic mixture and a chiral selector. The analytical databases affirm that 1, 2 dichloroethane is used as a non-aqueous (organic media) solvent and that 1,3-butyl-3-methylimidazole L-tryptophan ([Bmim] [Ltrp]) opted used as a specific chiral ionic liquid. The developed ionic liquid based chital hplc method was successfully applied to separation and purification of isomers of Besifloxacin in during the process industries in bulk drugs.
KEYWORDS:Besifloxacin; Chiral Ionic Liquid; Two-phase Separation; Zero-point energy
Download this article as:| Copy the following to cite this article: Shankar E. B, Naidu C. G, Devaraju S, Rao K. V, Ramachandra B, Rao Y. S, Marok S. S. Chiral Ionic Liquid-Based Vortex-Assisted Enantio-Separation of S-(+) and R-(-) Besifloxacin and Evaluation of Zero Point Energy by Two-Phase Liquid-Liquid Extraction. Orient J Chem 2024;40(1). |
| Copy the following to cite this URL: Shankar E. B, Naidu C. G, Devaraju S, Rao K. V, Ramachandra B, Rao Y. S, Marok S. S. Chiral Ionic Liquid-Based Vortex-Assisted Enantio-Separation of S-(+) and R-(-) Besifloxacin and Evaluation of Zero Point Energy by Two-Phase Liquid-Liquid Extraction. Orient J Chem 2024;40(1). Available from: https://bit.ly/491Zs41 |
Introduction
Fluoroquinolones are broad-spectrum antibacterial agents that are effective against both Gram-negative and Gram-positive microscopic organisms and are specifically used in the treatment of bacterial infections acquired in emergency clinics. BSFX hydrochloride (BSFX) is a brand-new fluoroquinolone developed for the treatment of ocular infectious diseases that affect the skin 1-3. The structural features of BSFX(BSFX) include an N-1 cyclopropyl group and a C-8 chloride substituent, which enable broad-spectrum action against virulent organisms as well as dealing with Gram-positive germs 4-6.
Synthetic DNA gyrase and topoisomerase-IV are restricted by the BSFX movement rules during the coiling and uncoiling of bacterial DNA. From the compound plan (Fig.1), it can be seen that the BSFX particle has one chiral center appearance and one set of enantiomer configurations. Clinical studies have shown that R-BSFX has greater antibacterial activity than the S-enantiomer 7 and that their physicochemical, pharmacological, and toxicological characteristics are very different in their normal structures 8,9. The authoritative authorities impose rigid requirements and demand critical analysis for assessing the safety and validity of chiral medications combined with pure enantiomers because antipodes are frequently regarded as contaminants. Therefore, as per ICH 6A guidelines 10, evaluation of S-isomer, viewed as degradation, is essential for quality control of R-BSFX.
A thorough reading analysis revealed that the assurance of BSFX in the areas of drug testing and administrative concerns was accounted for by a few logical tactics as well as Bioanalytical procedures. BSFX in natural liquids is guaranteed. GU used LC-MS/MS to develop a processable method for examining BSFX in rabbit plasma and intra-visual tissues 11. BSFX in human tears has been evaluated using an LC-MS approach by Arnold 12. An RP-HPLC technique with bright recognition for the quantitative assurance of BSFX in ocular solutions has been tested by Costa13. However, these methods are achiral and do not deal with the separation of BSFX’s R- and S-enantiomers separately.
Recently, Wang. screened various chiral fixed stages for the separation of BSFX enantiomers, including Chiral AGP, Crown Pak CR (+) (Daicel Chemical IND., Ltd., Japan), and Chiral CD-Ph. (Shiseido Co., Ltd., Japan), but they were unsuccessful in achieving palatable selectivity and significant results. Then, for separation and guarantee of the enantiomeric debasement of R-BSFX, they suggested pre-section derivatization using 2, 3, 4, 6-tetra-O-acetyl-beta-d-glucopyranosyl is thiocyanate14. In vitro, ex vivo, and in vivo strategies for BSFX HCl were developed by Kerem Polat. for the treatment of bacterial keratitis 15. There is currently no report available on BSFX ionic fluid extraction and the determination of zero-point energy using liquid extraction in two stages.
The current proposed ionic liquid based chiral HPLC assay method provides, the distribution strategies of enantiomers of BSFX are examined in a two-phase chiral ionic liquid-based liquid-liquid smaller-than-expected extraction structure. This structure is equipped with both conventional dissolvable, such as methanol, and aqueous media solvent (ionic liquid media), which contain CIL as chiral extractant. The technique for quantum physics calculations using Gaussian 16 programming and further verification using extraction tests of the CIL kind were initially developed concurrently. In addition to gathering BSFX enantiomers, this technique was developed with several different factors in mind, including standard dissolvable materials, chiral extractants, and including physical studies (pH) (effect of temperature on enantio-separation). Additionally, we performed a different extraction procedure (CIL media), which served as a significance of the use of CILs in the pharmaceutical industries.
Materials and Equipment
Chemical materials and Solvents
We used deionized water, ethanol, and diethyl amine from Sisco Research Laboratories Pvt. Ltd. in Mumbai, India; HPLC-grade methanol from SD Fine Chemicals in Mumbai, India; and n-hexane from Rankem in Mumbai, India. BSFX were obtained from a nearby collection unit in Hyderabad, India (Racemic mixture, purity >99 %; Fig.1). Was acquired from Sigma-Aldrich, India, and contains 1 butyl-3 methylimidazolium L-tryptophan ([BMIM] [Ltrp], 97%), 1 butyl-3 methylimidazolium L-phenylalanine ([BMIM] [L phe], 97%), and 1 butyl-3 methylimidazolium L-serinate ([BMIM][L ser], 97%).
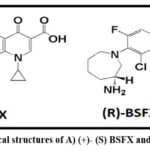 |
Figure 1: Chemical structures of A) (+)- (S) BSFX and B) (-)-(R) BSFX. |
Figure 2 depicts the compound evolution of CILs. Methanol (HPLC grade, 99.5%), dichloromethane (AR, 99.5%), and dichloroethane (AR, purity, 100%) were acquired from Merck in Bangalore, India. Trifluoroacetic acid (TFA, HPLC grade, 99.5%) and Sodium dihydrogen phosphate (NaH2PO4, AR, nearly 100 per cent) were bought from Rankem in Mumbai, India. N-Octanol (AR,100%), N-decanol (AR, 98%), ethanol (HPLC grade, 99.8%), phosphoric acid (H3PO4, AR, 85%), and sodium dihydrogen phosphate. We bought n-hexane (HPLC grade, 95%) from Sigma-Aldrich Pvt.Ltd,. By combining sodium dihydrogen phosphate, disodium hydrogen phosphate, and phosphoric acid, ionic pairing agents, H3PO4/NaH2PO4/Na2HPO4 in various pH ranges. The pH evaluation was carried out with modernized pH meter (Sisco Research Laboratories Pvt. Ltd., Mumbai, India).
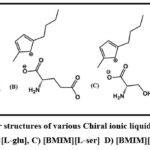 |
Figure 2: The molecular structures of various Chiral ionic liquids A) [BMIM][L‐phe], B) [BMIM][L-glu], C) [BMIM][L‐ser] D) [BMIM][L‐trp]. |
Equipment
HPLC equipment furnished with two LC-20AD pumps, a SPD-M20A diode cluster finder (PDA), a SIL-20AC autosampler, a DGU-20A5 degasser, a CTO-20 AC thermostat, and a CBM-20A correspondence module (all from Shimadzu, Kyoto, Japan) was utilized. The chromatographic data was recorded through an HP-Vectra (Hewlett Packard, Waldron, Germany) PC framework utilizing LC-Solution information-getting programming (Shimadzu, Kyoto, Japan). A polarimetric detector (IBZ Messtechnik GmbH, Hannover, Germany) was utilized to identification of the enantiomers. Chiralcel OD-H, Chiralcel OJ-H, and Chiralpak AD-H, Chiralpak IE-3 columns tried (250 × 4.6 mm, 3 μm) from Daicel Chemical Industries, Ltd. (Tokyo, Japan) were utilized for the currently proposed method.
Extraction experiments
Extraction process
Chiral ionic liquid (CIL) and a racemic mixture of BSFX were broken down using media with a specific pH of 0.29 molL-1 and a buffered media. Because BSFX dissolves poorly in water, it was decided to focus on the dissolvability by expansion of methanol 10% (v/v), which was chosen as a specified dissolvable solvent.
The aqueous stage containing the CIL medium was combined with an equivalent amount of pure natural dissolvable at a reasonable temperature, and the mixture was agitated for 2.5 hours until for the extraction. After, a base (limited quantity) of the CIL added to the BSFX enantiomers were using high-performance liquid chromatography (HPLC) for the separation. The chromatographic run is shown in Fig. 3.
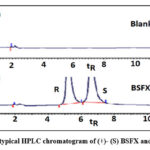 |
Figure 3: The typical HPLC chromatogram of (+)- (S) BSFX and (-)-(R) BSFX. |
Antipode extraction process
The antipode extraction process was initiated, which involved collecting the removed liquid stage, adding varying amounts of dichloromethane (DCM) in the proper amounts, and flustering the mixture at room temperature for 2.5 hours while continuously obtaining the opposite stage. After some time of adjustment and phase separation is takes place (intentionally isolated) aqueous phase, which is the stage that yields the most promising results and also completes the additional recovery via exhaustive ionic fluid (IL) without further detachment and decontamination of both pages. Fig. 4 shows a schematic representation of the extraction through phase media.
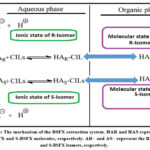 |
Figure 4: The mechanism of the BSFX extraction system. HAR and HAS represent the R-BSFX and S‐BSFX molecules, respectively. |
Analytical statistical evaluation methodology
The enantio-separation was accomplished using HPLC in conjunction with a second Chiralpak IE-3 section that included a non-movable amylose tris (3, 5 dichlorophenylcarbamate) chiral fixed stage and a 3micron silica phase that was identified and assessed. A dissolvable delivery system that operates on the same portable stage as per condition, contacting hexane media (with 0.1% Trifluoroacetic acid): ethyl alcohol (85:15, v/v) at a rate of 1 mL/min. 5 µL is the injection volume. The standard R-BSFX enantiomers were used to separate that the R-maintenance isomer’s in a mixture was not the same as that of the S-enantiomer, and the peaks of the R-BSFX and S-BSFX enantiomers were completely and specifically isolated.
The two enantiomers were measured using the conventional factual measurements of R-BSFX (A = 115.36C + 145.02, r2 = 0.9997, A and C are the factors) and SBSFX (A = 126.18C19.44, r2 = 0.9996). The linearity range for the finder reactions was 10-200 µg/mL, and the lowest feasible concentration for R- and S-BSFX was 0.05 and 0.14 µg/mL, respectively. The reproducibility of the purposeful enantioselectivity is approximately 2%, and the reproducibility of the dispersion coefficient was essentially seen inside 5%. The developed logical method was regarded as delicate and particular for the isomers in turn.
CIL-based two-phase separation mechanism
Figure 5 shows the stage-isolated phase (aqueous phase) consensus and response intentional extraction desired compound. The extraction of the BSFX enantiomer between the two phases , After the first phase separation consensus of the BSFX in the water phase, the next conversion was also evaluated through a balance between the CILs and the BSFX analytes, sub-atomic collaborations like electrostatic as well as hydrogen security connection and Van de Waals forces of attraction through the use of the following equations, the suggested study briefly displayed the measured functions.
kS =Cs,o/CS,W → (1)
kR =CR,O/CR,w → (2)
α=kS/kR → (3)
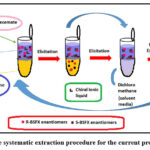 |
Figure 5: The systematic extraction procedure for the current proposed work. |
Results
In this proposed CIL method, quantum science assessments were carried out using Gaussian 16 programming as the primary device. Quantum mechanical science calculations address. Analyze the relevant calculations without even remotely associated constraints. Every aspect of the established factual bounds was handled under idealized settings, and all advanced initial calculations that could be performed were proposed carefully, which was important. These data are cross-checked using an appropriate factorial design approach. Table. I provide the change in energy values for both isomers.
Table 1: Calculated change in energy values of chiral ionic liquids.
|
Chiral Ionic Liquid (CIL) |
∆ER (kcal mol-1) |
∆Es (kcal mol-1) |
∆E (kcal mol-1) |
|
[BMIM][L-trp] |
-21.69 |
-19.58 |
2.11 |
|
[BMIM][L-glu] |
-25.13 |
-22.50 |
2.63 |
|
[BMIM][L-ser] |
-28.09 |
-29.87 |
-1.78 |
|
[BMIM][L-phe] |
-21.17 |
-19.74 |
1.43 |
Screening of CILs
First, we looked at the viable limits of Chiral Ionic Liquid (CIL) and looked at the energy barriers between each isomer and CIL. Results of the information inquiry in quantum physics. According to the higher E in direct estimation confirmations data listed in Table II, the two delegate frameworks have obtained the greater enantioselectivity as well as pit appropriation coefficient. This is likely because of their actual features of amino corrosive anion. The relationship between the differences in energy levels of extracted isomers is shown in Fig. 6.
Table 2: The Influence of different CILS on distribution coefficient and enantioselectivity.
|
Chiral Ionic Liquid (CIL) |
kR |
ks |
α |
|
[BMIM][L-trp] |
20.35 |
24.13 |
1.185 |
|
[BMIM][L-glu] |
19.87 |
22.45 |
1.129 |
|
[BMIM][L-ser] |
17.84 |
19.42 |
1.088 |
|
[BMIM][L-phe] |
20.69 |
24.81 |
1.199 |
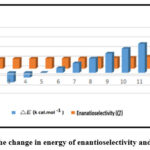 |
Figure 6: The change in energy of enantioselectivity and △E values. |
Influence of organic solvents
At ambient temperature, the currently suggested chiral ionic fluid-based enantio-separation of BSFX using several chiral extraction processes was examined. The impact of various solvents on distribution coefficient and enantioselectivity data are given in Table 3.
Table 3: The consequence of different solvents on distribution coefficient and enantioselectivity.
|
Solvents |
kR |
ks |
α |
|
Dichloroethane |
21.35 |
25.17 |
1.178 |
|
n-hexane |
18.97 |
22.95 |
1.209 |
|
n-decanol |
16.04 |
19.81 |
1.235 |
|
Dichloromethane |
36.14 |
43.57 |
1.205 |
|
n-octanol |
11.49 |
12.86 |
1.119 |
Influence of pH
In the suggested method, pH plays a crucial role. For both isomers, the appropriate pH value was verified in the range of 2–11. The calculated transportation information for the extracted isomers in both phases and reverse phase analysis. The pH of the fluid stage is distinctly impacted by the addition of specific additional substance focuses in the two stage extraction and is decreased with an increase in pH (it indicates connections between practical groups contained in the chiral selector). The representative impact of pH is depicted in Fig. 7,
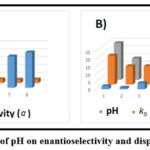 |
Figure 7: The impact of pH on enantioselectivity and dispersion coefficients. |
As a result, BSFX is a weakly corrosive drug with pKa values for both isomers. As pH increases, a large number of BSFX analytes transform into BSFX-separated isomers, which improves BSFX’s solubility in water and results in a significant decrease in the adsorption coefficient (in comparison to chiral selector). In the interim, the atomic BSFX compared and BSFX analytes have more interactions with the CILs. Results demonstrating interactions between BSFX isomers.
Influence of [BMIM] [L‐trp] concentration
The two enantiomers of coefficients significantly decreased with improved alteration of [BMIM] [Ltrp], a chiral selector, which was caused by a stronger bonds with the selective chiral ionic liquid. The BSFX data concentration is shown in Fig. 8.
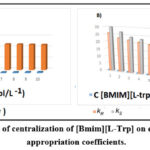 |
Figure 8: The impact of centralization of [Bmim][L‐Trp] on enantioselectivity and appropriation coefficients. |
Influence of Besifloxacin concentration
BSFX has a decreased dissolvability in the water phase, the [BMIM] [Ltrp] concentration ranges are 0.078-0.38 mmol/L in the liquid stage. The shift of mixture coefficients is brought about by the gradual expansion of the BSFX analytes in the usual stage, and the reduction of enantioselectivity is brought about by the presence of more nonselective extraction. Therefore, the higher enantioselectivity benefits from the lower initial concentration, and 0.24 mmol/L is chosen as the suitable fixation in the carefully determined extraction limit. The concentration profile is displayed in Fig.9.
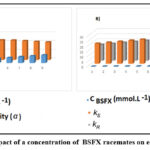 |
Figure 9: The impact of a concentration of BSFX racemates on enantioselectivity. |
Influence of temperature
Due to the significant insistence breaking point of [BMIM][Ltrp], which is illustrated in Fig.10,
the enantioselectivity plays an important role in the current extraction, which is from 0 to 25°C and shows 1.24 at RT. Due to the excessively high dispersion coefficients and minimum BSFX interactions in the aqueous stage, an expansion of enantioselectivity has been seen precisely when the temperature keeps rising over 35°C, which affects the extraction process. A reasonable extraction temperature is regarded as being at room temperature.
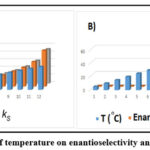 |
Figure 10: The impact of temperature on enantioselectivity and dispersion coefficients. |
Recycling of [Bmim] [L‐trp]
Reusing [Bmim] [Ltrp] the reuse of ionic liquid is essential for enabling a frequently occurring, cost-effective extraction procedure. The [Bmim] [Ltrp] was reused and the entire liquid stage was reused after extraction in this work, cutting off any further dividing and cleaning. Due to the more pronounced development coefficients distinguished between dichloromethane and dichloroethane, and the resulting higher reverse extraction rate, which relies on 98% achieved, dichloromethane was chosen as the proper conventional solvent in the contrary extraction procedure. Figure 11 illustrates the reused [Bmim] [Ltrp] holding fluid stage over five cycles.
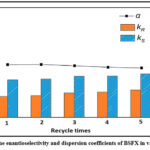 |
Figure 11: The enantioselectivity and dispersion coefficients of BSFX in various reuses. |
Discussion
In the present chiral method suggested that, a very effective chiral ionic liquid was designed for the particular interaction of racemic with chiral ionic liquids and two-phase liquid-liquid extraction to separate the BSFX enantiomers. The resulting analytical databases show that 1, 2 dichloroethane is employed as a non-aqueous (Organic media) solvent and that 1, 3-butyl-3-methylimidazole L-tryptophan ([Bmim][Ltrp]) is used as a particular chiral extractor. It might be helpful for the separation and purification process in the bulk industry as well as the research and development field.
Conclusion
Utilizing chiral ionic liquids and a vortex-aided technique, a unique, quick separation of the BSFX enantiomers was created along with an analysis of their zero-point energy. S-BSFX displays significant results in hospital for the clinical antibacterial activity, however the R-isomer has not demonstrated biological features with the relevant cells. The realistic values with substantial observations of purity about S-BSFX are displayed. The proposed method depicts the quantum physics calculations using Gaussian 16 programming and further verification using extraction tests of the CIL kind were initially developed concurrently. In addition to availing chiral drug enantiomers, this technique was developed with several different factors in mind, including standard dissolvable materials, chiral extractants, and headway studies (liquid stage pH) (effect of temperature on enantio-separation). Additionally, we performed a different extraction procedure (CIL media), which served as a notification of the use of CILs in the pharmaceutical industry.
Acknowledgements
The authors would like to express their gratitude to Vignan’s University, VIIT for their communication, persistent encouragement, and ongoing assistance in getting this study published in your esteemed journal.
Competing Interests
The Authors have declared that no competing interests for the disclosed work.
References
- Appelbaum, P.C; Hunter P.A.; The fluoroquinolone antibacterials: past, present and future perspectives; International Journal of Antimicrobial Agents, (2000); 16:5–15.
CrossRef - Smith, A; Pennefather P.M; Kaye; S.B, Hart, C.A.; Fluoroquinolones: place in ocular therapy. Drugs, (2001); 61:747–761.
CrossRef - Chang, M.H., Fung, H.B.; Besifloxacin: a topical fluoroquinolone for the treatment of bacterial conjunctivitis. Clinical Therapy, (2010); 32:454–471.
CrossRef - Haas; W. Pillar; C.M. Zurenko; G.E. Lee; J. C, Brunner, L.S; Morris, T.W; Besifloxacin, a novel fluoroquinolone, has broad spectrum in vitro activity against aerobic and anaerobic bacteria. Antimicrobial Agents Chemotherapy, (2009); 53:3552–3560
CrossRef - Haas, W, Pillar; C.M., Hesje, C.K; Sanfilippo; C.M., Morris, T.W, Bactericidal activity of BSFXagainst staphylococci, Streptococcus pneumoniae and Haemophilus influenza. Journal of Antimicrobial Chemotherapy, 2010; 65:1441–1447
CrossRef - Haas, W; Gearinger, L.S; Usner, D.W; Decory, H.H., Morris, T.W.; Integrated analysis of three bacterial conjunctivitis trials of BSFXophthalmic suspension, 0.6%: aetiology of bacterial conjunctivitis and antibacterial susceptibility profile. Clinical Ophthalmology, (2011); 5:1369–1379
CrossRef - Stephens, T.D; Bunde, C.J; Fillmore, B.J; Mechanism of action in thalidomide teratogenesis. Biochem Pharmacol, (2000); 59:1489–1499
CrossRef - Andrea, F; Michaela, V; Janka Mydlova, J.L.; Jan, K.; Interconversion of oxazepam enantiomers during HPLC separation. Determination of thermodynamic parameters. Journal of Liquid Chromatography and Related Technologies, (2006); 29:2889–2900
CrossRef - ICH Topic Q 6 a Specifications: test procedures and acceptance criteria for new drug substances and new drug products; chemical substances. http://www.ich.org/fileadmin/Public_Web_Site/ ICH_Products/Guidelines/Quality/Q6A/Step4/Q6Astep4.pdf. Accessed 6 Oct 1999.
- Gu, X.F.; Mao, B.Y; Xia, M., Yang, Y; Zhang, J.L.; Yang, D.S; Wu, W.X., Du, Y.X., Di, B., Su, M.X.; Rapid, sensitive and selective HPLC–MS/MS method for the quantification of topically applied BSFXin rabbit plasma and ocular tissues: application to a pharmacokinetic study. Journal of Pharmaceutical and Biomedical Analysis, (2016).; 117:37–46.
CrossRef - Arnold, D.R; Granvil, C.P.; Ward, K.W; Proksch, J.W.; Quantitative determination of Besifloxacin, a novel fluoroquinolone antimicrobial agent, in human tears by liquid chromatography–tandem mass spectrometry. Journal of Chromatography B, (2008); 867:105–110
CrossRef - Costa, M.C.N; Barden, A.T; Andrade, J.M.M., Oppe, T.P; Schapoval, E.E.S; Quantitative evaluation of BSFXophthalmic suspension by HPLC, application to bioassay method and cytotoxicity studies. Talanta, (2014); 119:367–374
CrossRef - Wang, Z; Wang, S; Zhu, F; Chen, Z; Yu, L; Zeng, S; Determination of enantiomeric impurity in BSFXhydrochloride by chiral high-performance liquid chromatography with precolumn derivatization. Chirality, (2012); 24:526–531.
CrossRef - Kerem Polat, H., Sibel Bozdağ, Pehlivan., Ceren, Özkul., Semih, Çalamak., Naile, Öztürk., Eren, Aytekin., Ayşegül, Fırat., Kezban, Ulubayram., Sibel, Kocabeyoğlu., Murat, İrkeç., Sema, Çalış.; Development of BSFXHCl loaded nanofibrous ocular inserts for the treatment of bacterial keratitis: In vitro, ex vivo and in vivo evaluation, International Journal of Pharmaceutics, (2020); 585: 119552.
CrossRef - ICH Q2 (R1) (2005) International conference on harmonization: validation of analytical procedures: test and methodology. http://www.ich.org/fileadmin/Public_Web_Site/ICH_Products/Guidelines/ Quality/Q2_R1/Step4/Q2_R1__Guideline.pdf. Accessed Nov 2005

This work is licensed under a Creative Commons Attribution 4.0 International License.









