Aqueous Medium Synthesis and Characterization of Mixed-Ligand Molecular Complexes of Manganese(III).
Department of Chemistry, Ramthakur College, Agartala 799002, West Tripura, India.
Corresponding Author E-mail: kantinathbhowmik@ gmail.com
DOI : http://dx.doi.org/10.13005/ojc/400125
Article Received on : 25 Sep 2023
Article Accepted on :
Article Published : 09 Jan 2024
Reviewed by: Dr. Aslam Sheikh
Second Review by: Dr. Tasneem Mohammed
Final Approval by: Dr. Charanjit Kaur
A fluoride-aided stabilization of manganese(III) was demonstrated by the preparation of unique complexes of the molecular mixed ligand fluoromanganate(III) from an aqueous medium. Complexes of mixed ligand fluoromanganate(III) were synthesized by reacting MnO(OH), 40% HF with nitrogen donor ligands that may function as neutral ligands, such as pyridine, ethylene diamine, or imidazole. The molecular complexes that were generated are stable and may be kept in polythene bags for extended periods of time without losing their properties. Consistency may be determined in a number of ways, including chemical analysis of the manganese oxidation state and periodic estimation of manganese and fluoride levels. The compounds dissolve slowly in water and partly in polar organic solvents. Element analyses, chemical oxidation state determination, FT-IR, electronic spectra, magnetic moment measurements at room temperature, thermogravimetric analysis (TGA/DTA), scanning electron microscopy (SEM), and cyclic voltammetry studies were used to characterize the complexes. The complexes produced probably have a distorted octahedral shape.
KEYWORDS:Aqueous Medium; Mixed-Ligand; Molecular Complex; Manganese(III); Stabilization
Download this article as:| Copy the following to cite this article: Bhowmik K. R. N. Aqueous Medium Synthesis and Characterization of Mixed-Ligand Molecular Complexes of Manganese(III). Orient J Chem 2024;40(1). |
| Copy the following to cite this URL: Bhowmik K. R. N. Aqueous Medium Synthesis and Characterization of Mixed-Ligand Molecular Complexes of Manganese(III). Orient J Chem 2024;40(1). Available from: https://bit.ly/3NXRgcx |
Introduction
Many redox enzymes 1-3 include manganese as an essential cofactor due to the metal’s ability to exist in many oxidation states. Since it is challenging to stabilize Mn(III) in aqueous medium4, the tri-positive state of manganese deserves special consideration due to its biochemical importance in various redox functions1- 3. Stable manganese(III) building blocks have been proven to be a helpful precursor for single-molecule magnets5, and it has been established that some manganese(III) complexes exhibit distinctive magnetic and structural properties6-8. Manganese(III) has the ability to undergo disproportionation in aqueous medium and has a fairly oxidizing power. These two characteristics make studying the coordination chemistry of manganese(III) challenging. Stable solid manganese(III) complexes are difficult to isolate for this reason. However, the formation of complexes9 is expected to lower the reduction potential of the Mn(III)/Mn(II) pair and thereby stabilize the manganese(III). Therefore, the determination of suitable ligands and sufficient techniques for the generation of stable manganese(III) complexes from aqueous solution is one of the most important prerequisites for future study. Few investigations have been carried out on molecular mixed-ligand fluoro complexes of manganese(III) with nitrogen donor ligands 10-13. Our interest in fluoro chemistry for manganese(III) led us to wonder about the possibility of synthesizing and studying molecular mixed-ligand fluoro complexes of manganese(III) containing nitrogen donors. This motivated the synthesis and characterization of mixed-ligand fluoromanganese(III) molecular complexes containing nitrogen donor co-ligands.
Experimental
Materials
The reagent grade chemicals were utilized for all the compounds. By the oxidation of Mn(OH)2 with hydrogen peroxide, the compound MnO(OH) was produced14.
Synthesis of molecular mixed-ligand fluoro complexes of manganese(III), [Mn(Py)2F3(H2O)], 1, (Py = pyridine), [Mn(en)F3(H2O)].3H2O, 2, (en=ethylenediamine) and [Mn(Imd)2F3(H2O)], 3, (Imd =imidazole)
General Procedure
An amount of 0.89 gram (10.11 mmol) of freshly prepared MnO(OH) was dissolved in 2.0 ml (40.00 mmol) of 40% HF with stirring. To the clear solution added 1.2 cm3 of pyridine (12.65 mmol)/ 0.7 cm3 of ethylene diamine (11.66 mmol)/ 0.772gram of an aqueous solution of imidazole (11.36 mmol) and stirred for about 20 min. Solid Na2CO3 was added to the newly produced reaction mixture to maintain pH ca.6, and the resultant mixture was then stirred for another 10 minutes. To the resultant solution added an equivalent amount of pre-cooled acetone, when a pink microcrystalline product was obtained. The compound was collected by filtration, washed 3-4 times with a very little amount of acetone and finally dried in vacuo over conc.H2SO4. There were several efforts made, but none of them were successful in obtaining single crystals suitable for X-ray diffraction studies.
Physical Measurements
Infrared spectra were recorded as KBr discs using a Perkin Elmer Spectrum100 FT-IR spectrometer. Perkin-Elmer model Lamda-25 was used to record the electronic spectra. The magnetic susceptibility measurements were carried out by Gouy’s method using Hg[Co(NCS)4] as standard. A Perkin Elmer Pyris Diamond model was utilized for TGA and DTA investigations at a heating rate of 5oC/min in N2 atmosphere. LEO1430VP scanning electron microscopes with energy-dispersive X-ray detectors were used for SEM characterization. Studies of cyclic voltammetry were carried out using an Electrochemical Analyzer apparatus type CH620A.
Elemental Analyses
Manganese was estimated volumetrically by the complexometric titration of EDTA15a , using Erio-T as an indicator. Volhard’s method 15b was used to determine the amount of fluoride contents present in each of the compounds. The percentages of carbon, hydrogen, and nitrogen were calculated using microanalytical methods. (The results of the analysis are shown in Table 1.)
Table 1: Analytical data for the complexes (1-3)
|
Compound |
Yield (%) |
Found (Calcd.) (%) |
||||
|
Mn |
F |
C |
H |
N |
||
|
[Mn(Py)2F3(H2O)], 1, (Py= pyridine) |
75% |
19.07 (19.09) |
19.76 (19.79) |
41.62 (41.66) |
4.16 (4.2) |
9.69 (9.72) |
|
[Mn(en)F3(H2O)].3H2O, 2, (en=ethylenediamine) |
77% |
22.51 (22.54) |
23.32 (23.36) |
9.81 (9.83) |
5.75 (5.78) |
11.43 (11.47) |
|
[Mn(Imd)2F3(H2O)], 3, (Imd=imidazole) |
79% |
20.87 (20.83) |
21.57 (21.59) |
13.67 (13.63) |
3.05 (3.03) |
21.19 (21.21) |
Chemical determination of the oxidation state of manganese
The oxidation state of manganese in the newly synthesized compounds containing trivalent manganese was determined iodometrically by treating a freshly prepared ice-cold solution of potassium iodide acidified by dilute sulfuric acid with the compound followed by titrating the liberated iodine against a standard solution of sodium thiosulfate.
Results and Discussion
Synthesis
A problem associated with the synthesis of manganese(III) compounds from aqueous media is the tendency of the metal ion to be disproportionate to manganese(IV) and manganese(II)4. It was anticipated that the problem associated with the synthesis of manganese(III) complexes from an aqueous medium may be encountered by carrying out the reaction in an acidic medium in presence of fluoride ions. This was because fluoride ions have a notable stabilizing effect on manganese (III). Stable molecular mixed ligand fluoro complexes of manganese(III) were expected to be produced from an aqueous medium using this method. Accordingly, synthetic strategy was chalked out and syntheses of molecular mixed ligand fluoro complexes of manganese(III) containing pyridine, ethylene diamine, and imidazole as nitrogen donor co-ligands were accomplished from the reaction of freshly prepared MnO(OH), 40% HF and the corresponding nitrogen donor co-ligands at pH ̴ 6 in presence of Na2CO3 solution. Slow addition of pre-cold acetone to the reaction mixture facilitated precipitation of the pink microcrystalline compounds in reasonably good yield.
Thus it is evident from the foregoing discussion that fluoride ion can stabilize the manganese(III) in aqueous media in presence of different co-ligands and allowed the formation of new mixed ligand fluoro complexes of manganese(III) containing nitrogen donor co-ligands from aqueous medium.
Magnetic Measurements
The results of magnetic susceptibility measurements at room temperature (300K) showed that the magnetic moments of complexes 1, 2 and 3, to be 4.95, 4.82 and 5.1 B.M. respectively. The values are typical for a high spin d4 manganese(III) ion system.The newly produced molecular mixed ligand fluoro complexes of manganese(III) lack the antiferromagnetism of binary fluoromanganate(III), K2[MnF5] 6,7.
Electronic Spectra
The electronic spectra of complexes 1, 2 and 3, were recorded in DMSO solution, the main features of these spectra are shown in Table 2. In case of complex 1, absorption bands were observed at ca.25,600 cm-1, ca.19,200 cm-1and ca.16,100 cm-1. The observed bands were assigned to 5B1g→5Eg, 5B1g→5B2g and 5B1g→5A1g modes of transitions respectively 16,17. Absorptions for, complex 2, were observed at ca. 23,800 cm-1 and ca.15,600 cm-1, assigned as 5B1g→5Eg and 5B1g→5A1g transitions, and the transition of complex 3, exhibits bands at ca. 25,600 cm-1, 19,200 cm-1 and ca. 15,100 cm-1, were assignable to the 5B1g→5Eg, 5B1g→5B2g and 5B1g→5A1g transition modes. The observed spectral pattern is characterized by the presence of high spin manganese(III) ion and indicates appreciable splitting of the 5Eg of manganese(III) ground state, leading to a distorted octahedral environment around manganese(III) centre 16,17 in the complex species.
Table 2: The Values of Magnetic Moment and Electronic Spectral Data for the Complexes (1-3).
|
Compound |
The Values Magnetic Moment at 300K, μB |
Positions of Electronic Spectral Band (cm-1 ) |
||
|
5B1g ® 5A1g |
5A1g ® 5B2g |
5B1g ® 5Eg |
||
|
[Mn(Py)2F3(H2O)], 1, (Py=pyridine) [Mn(en)F3(H2O)].3H2O, 2, (en=ethylenediamine) [Mn(Imd)2 F3(H2O)], 3, (Imd=imidazole) |
4.95
4.82
5.1 |
16,100
15,600
15,100 |
19,200
–
19,200 |
25,600
23,800
25,600 |
Infrared Spectra
The FT-IR spectra of the complexes displayed well resolved spectral patterns; the main features of these spectra are shown in Table 3. The infrared spectrum of compound 1, exhibit the typical absorptions owing to the coordinated fluoride and pyridine molecule. Solid state IR spectrum of compound 1, show strong absorptions at 1638 cm-1 that may be attributed to ν (C=C), stretching vibrations of ν C=C bond of pyridine. Characteristic out of plane and in-plane deformation bands for free pyridine are observed at 604 and 403 cm-1 18 . In the case of, 1, these bands were seen at 625 and 437 cm-1. In contrast to the values reported for that of the free pyridine, these values were shifted to a higher frequency, which suggests coordination of pyridine nitrogen to the metal centre. The Mn-N stretching mode originating from the coordinated N-heterocyclic ligand, pyridine observed at ca. 403 cm-1, providing further evidence of the occurrence of coordinated pyridine in the compound. Appearance of bands at around 571, 593 cm-1 have been attributed to the coordinated fluoride ligand 19, ν (Mn-F). The appearance of broad band at around 3460 cm-1 has been assigned as ν (OH). The rocking mode of vibration, ρr(H2O) of coordinated water molecule 20 is observed at ca.734cm-1.
The IR spectrum of compound 2 shows characteristics absorptions due to coordinated ethylenediamine, coordinated fluoride and coordinated as well as lattice water. The major bands coming from coordinated ethylenediamine are observed at 1592, 1511, 1351 and at 1034 cm-1 assigned to δ(NH2), δ(CH2) and ν (skeletal) modes of vibrations of coordinated ethylene diamine 21,22. The observed frequencies and pattern originating from ethylenediamine suggest that ethylenediamine ligand occurs as bidentate one in the compound under discussion. The spectral pattern observed in the present case is not as simple as the one that would be observed if the ethylenediamine ligand was to be present as trans bridging group 23, leads us to conclude that ethylene diamine binds the manganese centre as a bidentate chelate and does not occur as a bridging group. The other important absorptions arising from coordinated ethylenediamine were observed in the range 3011-2893 cm-1 assigned as ν(NH) mode of vibration. The reduction of ν(NH) compared to that of free ethylenediamine also suggests coordination of ethylenediamine to the metal centre involving its nitrogen atoms. Fortunately, the appearance of a band with medium intensity at 808 cm-1 has been assigned to the rocking mode of vibration of coordinated water. This is especially significant since compound, 2, contains both coordinated as well as lattice water. The information for the presence of lattice water that may be obtained from ν(OH) and δ(H-O-H) observed at ca. 3438 and 1631cm-1, is not very significant as per as the distinction between two kinds of water molecules is concerned. Fortunately the occurrence of a rocking mode of water as mentioned above support the presence of coordinated water in the compound under discussion.
Table 3: Structurally Significant IR bands for the Complexes (1-3).
|
Compound |
Band position (cm-1) |
Assignment |
|
[Mn(Py)2F3(H2O)], 1, (Py=pyridine) |
3460 1638 625
437
734 593, 571 403 |
ν(OH) ν(C=C) Out of plane deformation of pyridine In plane deformation of pyridine ρr(H2O) ν(Mn-F) ν(Mn-N) |
|
[Mn(en)F3(H2O)].3H2O, 2, (en=ethylenediamine) |
3438 1631 3011-2893 1592 1511,1351 1034 808 578 |
ν(OH) ν(H-O-H) ν(NH) δ(NH2) δ(CH2) ν(skeletal) ρr(H2O) ν(Mn-F) |
|
[Mn(Imd)2F3(H2O)], 3, (Imd =imidazole) |
1600-1300 – – 745 591 418 |
C-H vibration N-H vibration Imidazole ring vibration ρr(H2O) ν(Mn-F) ν(Mn-N) |
The main characteristics of compound compound 3’s infrared spectrum were absorptions due to coordinated imidazole, fluoride and water molecule. Bands observed in the region 1600-1300 cm-1 owe their origin to C-H, N-H and ring vibrations of coordinated imidazole 24. Terminal coordination of the fluoride ligand was ascertained from the appearance of band at 591 cm-1. Metal nitrogen stretching vibration, ν(Mn-N), was observed at ca. 418 cm-1 which support the concept that the imidazole molecule’s nitrogen atom is coordinated to the Mn(III) centre 25. A notable feature of the IR spectrum of compound 3, is the band at ca. 745 cm-1 which has been assigned to the rocking mode, ρr(H2O) of coordinated water. This is especially significant since the information obtainable from the ν(OH) and δ(H-O-H) positions are not very helpful for ascertaining the nature of water molecule present in the compound.
Thermo Gravimetric studies
Thermo gravimetric analysis (TGA/DTA) of molecular complex, 2, was carried out in an environment of N2 at temperature ranging from 40-1000oC (Figure1). Thermogram of compound 2, showed an endothermic peak between 227-246oC, corresponding to the loss of weight of 23.21%, which was caused by the release of three molecules of lattice water (calcd. loss 22.13%) per formula weight of the compound. The relatively higher temperature required for the removal of coordinated water molecules probably be due to involvement of water molecule in hydrogen bonding interactions in the crystal lattice. The dehydrated material slowly disintegrates after being heated to over 246 oC, most likely due to the thermal degradation of organic ligands and the loss of coordinated water; however, the amount of disintegrated fragments could not be estimated due to the continuous weight loss in the process.
 |
Figure 1: TGA /DTA curve for compound 2. |
SEM and Energy dispersive X-ray (EDX)
The surface layers of the molecular complex, 2, were seen using SEM is homogeneous as shown in Fig. 2.
 |
Figure 2: Scanning electron micrograph of compound 2. |
Energy dispersive X-ray spectroscopy was performed on the molecular complex, 2, which provides in situ chemical investigation of the bulk, has clearly showed Mn, F, C, O, N, etc. as the constituent elements of the newly synthesized complexes (Fig.3). In addition, the EDX spectral data that was collected on the composition of the compound was found to be in good agreement with the values obtained from the elemental analyses.
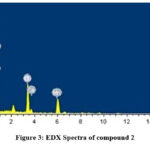 |
Figure 3: EDX Spectra of compound 2 |
Electro chemical study
The electrochemical property of the complex 2 has been studied by cyclic voltametry as the compound is appreciably soluble in DMSO. In the case of other two molecular complexes 1 and 3, their electrochemical behaviour could not be determined because of their partial solubility in DMSO or DMF. Cyclic voltametry of complex 2 shows an irreversible behaviour (Fig.4) may be assigned to a Mn(III)/Mn(II) couple based on the observed Eo and ∆Ep values of 0.15V, 1.7V, when scanned at a rate of 0.1V s-1.
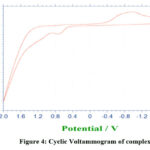 |
Figure 4: Cyclic Voltammogram of complex 2 |
Conclusion
Therefore, in order to reach a conclusion, it is essential to emphasize the generation of molecular mixed ligand fluoro complexes of manganese(III) including nitrogen donor ligands by carefully choosing reaction conditions in an aqueous medium. In this study, it was shown that all of the compounds remain stable for long periods of time if they are kept dry. When compared to the binary fluoro complexes of manganese(III), specifically A2[MnF5], the newly synthesized compounds described in the present work have normal magnetic moments, as is expected for d4 manganese(III) systems, whereas the A2[MnF5] compounds exhibit strong antiferromagnetic interactions. Electrochemical study of compound 2 in DMSO solution show irreversible redox behavior due to presence of Mn(III)/Mn(II) couple. The electronic spectrum analysis shows that an appreciable splitting of 5Eg ground state of manganese(III) in the complex. Each of the newly synthesized complexes may have a distorted octahedral structure.
Acknowledgement
The author is thankful to Prof. A. T. Khan, Department of Chemistry I.I.T, Guwahati for help in recording of magnetic susceptibility. Author is also thankful to Prof. N. S. Islam, Tezpur University for carrying out SEM, EDX studies and to Prof. R. N. Dutta Purkayastha, of Tripura University, Agartala for his help and cooperation.
Conflicts of interest
There are no conflicts to declare.
References
- Larson, J. E.; Pecoraro, V. L., “Manganese Redox Enzymes”, VCH Publishers, New York, 1992, p. 1; Warrishi, H.; Khadar, V.; Gold, M. H., Biochem. 1989,28, 6017-6023; Law, N. A.; Caudle, M. T.; Pecoraro, V. L., Adv. Inorg. Chem. 1998, 46, 305-440; Leto, D. F.; Jackson, T. A., J. Biol. Inorg. Chem. 2014, 19, 1-15.
- Sarsour, E. H.; Kalen, A. L.; Goswami, P. C., Antioxidants and Redox Signaling, 2014, 20(10), 1618-1627; Zhu, W.; Richards, N. G. J., Essays Biochem. 2017, 61(2), 259-270; Zou, X.; Ratti, B. A.; O’Brien, J. G.; Lautenschlager, S. O.; Gius, D. R.; Bonini, M. G.; Zhu, Y., J. Bioen. Biomem. 2017, 49, 325-333.
CrossRef - Christou, G., Acc. Chem. Res. 1989, 22, 328-335; Signorella, S.; Hureau, C., Coord. Chem. Rev.2012, 256(11-12), 1229-1245; Schmidt, S. B.; Husted, S., Plants. 2019, 8, 381-396.
CrossRef - Cotton, F. A.; Wilkinson, G.,“Advanced Inorganic Chemistry – A comprehensive Text”, 4th ed., Wiley- Interscience, NewYork, 1980, p. 741-742; Nath Bhowmik, K. R.; Paul, P.; De, A. K.; Roy, S.; Dutta Purkayastha, R. N., J. Ind. Chem. Soc. 2011, 149, 190-201.
- Inglis, R.; Stoumpos, C. C.; Prescimone, A.; Siczek, M.; Lis, T.; Wernsdorfer, W.; Brechin, E. K.; Milios, C. J., Dalton Trans. 2010, 39, 4777-4785; Dhers, S.; Wilson, R. K.; Rouzieres, M.; Clerac, R.; Brooker, S., Cryst. Growth Des. 2020, 20(3), 1538-1542.
CrossRef - Massa, W., Inorg. Nucl. Chem. Lett. 1977, 13, 253-258; Liu, Q.; Yu, L.; Wang, Y.; Ji, Y.; Horvat, J.; Cheng, M. L.; Jia, X.; Wang, G., Inorg. Chem. 2013, 5(6), 2817-2822; Orio, M.; Pantazis, D. A.; Petrenko, T.; Neese, F., Inorg. Chem. 2009, 48(15), 7251-7260.
CrossRef - Milios, C. J.; Presimone, A.; Mishra, A.; Parson, M.; Wernsdofer, W.; Christou, G.; Perlepes, S. P.; Brechin, E. K., Chem. Commun. 2007, 53, 153-155; Hajikhanmirzaei, L.; Safaei, E.; Wojtzak, A.; Lajlicic, Z., Inorg. Chim. Acta. 2015, 430, 125-131; Zhang, H.; Li, C-K.; Liu, J.; Wang, Z-B.; Liu, L-Y.; Lu, Z-X.; Huang, Y-G., Res. Chem. 2023, 6, 101065
- Zhengliang, L.; Yhan, M.; Pen, F.; Gao, S.; Zhang, D.; Daoben, Z., Inorg. Chem. 2006, 45, 3538-3548and references therein; Li, Y.; Wernsdorfer, W.; Clerac, R.; Hewitt, I. J.; Anson, C. E.; Powell, A. K., Inorg. Chem. 2006,45, 2376-2378 and reference therein.
- Wood, C. W.; Holliday, A. K., Inorganic Chemistry, 3rd ed., Butterworths, London, 1967, p. 336; Alvarez, L.; Suarez, S. A.; Bikiel, D. E.; Reboucas, J. S.; Batinic-Haberle, I.; Marti, M. A.; Doctorovich, F., Inorg. Chem. 2014, 53(14), 7351-7360.
CrossRef - Rother, G.; Stief, R.; Bentrup, U.; Massa, W., J. Fluor. Chem. 2011, 132(10), 740-746.
- Elias, C.; Fuentes, J.; Nunez, P.; Rodriguez, V. D.; Jacobs, U.; Massa, W., Z. Anorg. Allg. Chem. 1998, 624, 2001-2006; Darriet, J.; Massa, W.; Pebler, J.; Stief, R., Solid State Sciences. 2002, 4, 1499-1508.
- Bhattacharjee, M. N.; Chaudhuri, M. K.; Dutta Purkayastha, R. N., Inorg. Chem. 1989, 28, 3747-3752.
CrossRef - Biju, A. R.; Rajasekharan, M. V., J. Mol. Struc. 2008, 875, 456-461.
CrossRef - Nath Bhowmik, K. R.; De, A. K.; Dutta Purkayastha, R. N., J. Ind. Chem. Soc. 2012, 89, 177-187 and references therein.
- (a) Vogel, A. I., “A Text Book of Quantitative Inorganic Analysis”, 3rd ed., Longmans, Green and Co., New York, 1962, p. 434; (b) Vogel, A. I., “A Text Book of Quantitative Inorganic Analysis”, 3rd ed., Longmans Green and Co., New York, 1962, p. 269.
- Kumar, G.; Devi, S.; Johari, R., J. Chem. 2012, 9(4), 2255-2260.
CrossRef - Lever, A. B. P., “Inorganic Spectroscopy”, Elsevier, Amsterdam, 1984; Sharma, V. K.; Srivastava, S., Turk. J. Chem. 2006, 30, 755-767.
- Dey, D.; Roy, S.; Purkayastha, R. N. D.; Pallepogu, R.; Male, L.; Mckee., V., J. Coord. Chem. 2011, 64, 1165-1176 and references therein.
CrossRef - Nakamoto, K, “Infrared and Raman Spectra of Inorganic and Coordination Compounds”, 4th ed., Wiley – Interscience, London, 1986, p. 324.
- Nakamoto, K. Infrared and Raman spectra of inorganic and coordination compounds: Part A: Theory and applications in inorganic chemistry. Wiley Publications, New York City, 2008.
CrossRef - Singh, N. K.; Bharty, M. K.; Kushawaha, S. K.; Dulare, R.; Butcher, R. J., Transition Met. Chem. 2010, 35, 337-344.
CrossRef - Jaiswal, D.; Yadava, S., Oriental Journal of Chemistry. 2018, 34(6), 2867-2871.
CrossRef - Bhattacharjee, C. R.; Goswami, P.; Mondal, P., J. Coord. Chem. 2010, 63(11), 2002-2011.
CrossRef - Archer, S. J.; Auf der Heyde, T. P. E.; Foulds, G. A.; Thornton, D. A., Trans. Met. Chem. 1982, 7, 59-62.
CrossRef - Nakamoto, K., “Infrared and Raman Spectra of Inorganic and Coordination Compounds”, 4th ed., Wiley – Interscience, London, 1986, p. 208.

This work is licensed under a Creative Commons Attribution 4.0 International License.










