Amla Fruit Extract: Green and Natural Catalyst for the Synthesis of Acylated Derivatives via Microwave Technique
Zeenath Unnisa Begum1 , Tasneem Mohammed2
, Tasneem Mohammed2 , Mohammadi Begum1
, Mohammadi Begum1 and Syeda Sameena Aziz1*
and Syeda Sameena Aziz1*
1Department of Chemistry, Anwarul Uloom College, New Mallepally, Hyderabad – 500001, India.
2Department of General Sciences, Ibn Sina National College for Medical Studies, Jeddah - 21418, Saudi Arabia.
Corresponding Author E-mail: ssaauc@gmail.com
DOI : http://dx.doi.org/10.13005/ojc/400115
Article Received on : 15 Dec 2023
Article Accepted on :
Article Published : 16 Feb 2024
Reviewed by: Dr. Abdellah Zeroual
Second Review by: Dr Mohamed Riad Fouad
Final Approval by: Dr. Malinee Sriariyanun
The current work demonstrates Amla fruit extract efficiency as a promising green catalyst for microwave assisted acylation processes. This is an attempt to recreate the ancient acylation procedures that used metal catalysts. The results obtained for the synthesis of naphthalene-2-yl-acetate, N-(2-nitrophenyl)acetamide, N-phenylacetamide, 1-phenylpropane-1,2-dione, acetic benzoic anhydride, N-acetylbenzamide, phenyl acetate (A1-A7) in the presence of amla juice as a green catalyst were exemplary which determines that the efficacy of the natural amla juice extract proved to be an excellent alternative catalyst. These reactions will represent a significant breakthrough in chemical synthesis, particularly in the acylation of organic molecules with potential biological activity.
KEYWORDS:Acetylation; Amla Juice; Green Synthesis; Microwave
Download this article as:| Copy the following to cite this article: Begum Z. U, Mohammed T, Begum M, Aziz S. S. Amla Fruit Extract: Green and Natural Catalyst for the Synthesis of Acylated Derivatives via Microwave Technique. Orient J Chem 2024;40(1). |
| Copy the following to cite this URL: Begum Z. U, Mohammed T, Begum M, Aziz S. S. Amla Fruit Extract: Green and Natural Catalyst for the Synthesis of Acylated Derivatives via Microwave Technique. Orient J Chem 2024;40(1). Available from: https://bit.ly/48pz5DP |
Introduction
Microwave-assisted chemistry has become more popular in organic synthesis over the years 1. In recent years, synthetic organic chemists have used ultrasound to improve conventional thermal heating for organic processes by decreasing reaction times and/or increasing yields, to promote novel reactions because it is significantly cleaner, greener, and more environmentally friendly 2-4. The N-acylation reaction is most frequently used in organic synthesis, pharmaceutical, biological, and agricultural sectors. Nowadays, it is vital to make chemistry safer for its practitioners for reasons of environmental safety and sustainability 5.
Researchers have already thoroughly investigated and recorded a wide range of “Microwave assisted” responses 6-10. However, the solvents or catalysts utilized so far for those “Microwave assisted reactions,” such as Pd (II) 11, FeCl3 12, CaCl2 13, and ZnI2 14, are hazardous, unfavorable to the environment, and toxic to human health. Natural chemicals are increasingly being used as a catalyst in the acylation reaction, and also in the synthesis of various organic compounds. Thus, the use of natural materials and ecofriendly practices has opened a new route to green chemistry. The energy efficiency of microwave (MW) irradiation and its capacity to accelerate chemical processes have led us to consider using this approach (MW irradiation) as a viable tool for our green acylation procedures. Tanay Pramanik, and Poulami Maji synthesized dihydropyrimidinones derivatives bearing both electron rich as well as electron deficient aromatic aldehydes by ultrasound irradiated Biginelli reaction in amla juice, lime juice and orange juice medium 15.
One of the primary advantages of using MWs as an energy source in chemical reactions is that the energy is created directly in the sample through absorption. Conventional thermal techniques such as sand baths, electric heating, oil baths, or heating jackets are no longer required. As a result, the process is more energy efficient because heating the entire system is unneeded. Another advantage is that MWs penetrate the sample, transferring heat faster than other methods in which heat is transported by convection from the surface and is highly dependent on the material’s inner thermal conductivity. The temperature profile is inverted as MWs penetrate the sample, and samples are efficiently heated from the inside to the outside 16.
The natural fruit extracts are fully environmentally safe, sustainable, ecologically sound, and non-hazardous in nature. At room temperature, some common fruit juices, such as Phyllanthus emblica Linn (amla), orange, and lime juices, have already been proved to be suitable as green reaction vehicle for the Biginelli reaction 17. Other juices like Cocos nucifera L. juice (coconut juice), Solanum lycopersicum L. juice (tomato juice), Vitis vinifera L. juice (Lemon juice), and Citrus limetta (musambi juice), have been used for synthesis of substituted dihydropyrimidinones derivatives, isoxazole derivatives and bioactive imidazoles respectively 18-20. Citrus juice as a new, efficient, environmentally friendly, and natural catalyst has been reported for substituted enaminones 21. Amla fruit extract was employed for production of 2-aryl-1,3-benzoxazole from ortho-Amino phenol and substituted aryl aldehydes by microwave irradiation22. A different study describes a low-cost, green production of AgNPs utilizing Phyllanthus emblica fresh fruit extract23.
Microwave assisted acylation synthesis in amla fruit juice medium is expected to be much more efficient, faster, and cleaner than room temperature synthesis, but our extensive literature search revealed that the researcher has yet to attempt to synthesize acylated derivatives in fruit juice medium using microwave (MW) irradiation. So, based on the aforementioned research gap, the primary goal of this work is to synthesize a series of acylated derivatives using a range of aromatic compounds in presence of nature derived catalyst and green solvent isopropyl alcohol.
Experimental section
General method for the preparation of acylated derivatives (A1-A7)
Preparation of Catalyst
250gm of amla (Indian goose berry) purchased from a supermarket was well cleaned before being coarsely grinded in a food processor and squeezed with the help of muslin cloth.100ml of pure extract were produced by the procedure. The amla fruit extract (AFE) was collected in a bottle and stored in the refrigerator to maintain its freshness and for further usage as a catalyst.
Procedure for Acylation
An equimolar mixture of the substrate and acetyl chloride were taken in isopropyl alcohol along with and few drops of amla fruit extract as green catalyst and was subjected to microwave irradiation at room temperature 273K for 2-8 minutes. The acylated products obtained (Figure 1) were characterized by melting point, boiling point, Rf value, IR, 1H-NMR, and mass spectra.
Characterization data of products
Naphthalene-2-yl-acetate (Compound A1)
Melting point: 67 °C (Reported 68-70 °C)
IR (neat): 1270, 1410-1260 cm-1.
1H-NMR (400 MHz, DMSO–d6): δ 7.263 (s, 1H), 7.694, 7.156, 7.128, 7.121 (d, 4H), 7.767, 7.434, 7.333 (t, 3H).
N-(2-Nitrophenyl) acetamide (Compound A2)
Melting point: 215 °C (Reported 212-216 °C)
IR (neat): 1621, 1680-1630cm-1
1H-NMR (400 MHz, DMSO–d6): δ 7.264 (s, 1H), 8.133, 8.111, 7.381, 7.343 (d, 4H), 7.364, 6.709 (t, 2H).
N-phenyl acetamide (Compound A3)
Boiling point: 304 °C (Reported 304 °C)
IR (neat): 1680, 1680-1630cm-1
1H-NMR (400 MHz, DMSO–d6): δ 2.615, 7.263 (d, 1H), 7.978, 7.485 (d, 2H), 7.962, 7.590, 7.572, 7.553 (t, 4H).
1-phenylpropane-1,2-dione (Compound A4)
Boiling point: 103 °C (Reported 103-105 °C)
IR (neat): 1683, 1740-1720 cm-1
1H-NMR (400 MHz, DMSO–d6): δ 2.353, 1.309 (d, 2H), 2.044 (t, 2H), 1.08 (q, 1H), 5.52, 2.83, 0.64, 0.63, 2.25, 0.33, 0.20 (m, 7H).
Acetic benzoic anhydride (Compound A5)
Melting point: 98 °C (Reported: 93-96 °C)
IR (neat): 1682, 1815-1790 cm-1
1H-NMR (400 MHz, DMSO–d6): δ 4.478, 7.250 (s, 2H), 8.139, 7.498, 7.459 (d, 3H), 8.123, 7.631, 7.616, 7.606 (t, 4H).
N-Acetylbenzamide (Compound A6)
Melting point: 119 °C (Reported: 117 °C)
IR (neat): 1652, 1680-1630 cm-1
1H-NMR (400 MHz, DMSO–d6): δ 7.463, 7.263 (s, 2H), 7.848, 7.829, 7.616 (d, 3H), 7.534, 8.109 (t, 2H).
Phenyl acetate (Compound A7)
Boiling point: 196 °C (Reported: 196 °C)
IR (neat): 1472, 1410-1260 cm-1.
1H-NMR (400 MHz, DMSO–d6): δ 2.098, 2.309 (s, 2H), 7.246, 6.840 (d, 6H), 7.259, 7.219, 6.925 (t, 3H), 2.12 (m, 1H).
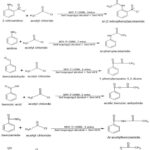 |
Figure 1: Synthetic route for derivatives A1-A7. |
Results and discussion
The results of acylation are summarized in Table 1. The reaction was tested in AFE under microwave irradiation, and it was found to be the most promising catalyst for the planned acylation processes of a variety of aromatic compounds. Acylated products were produced within minutes with good yields. It is noteworthy to note that neither the aromatic ring nor the substituted group of the aromatic system, such as the nitro group, was acylated.
The amla fruit juice extract catalyst’s efficacy was shown during the acylation processes of few common aromatic compounds. Excellent results were obtained with good yields in a very short period, and the reaction went swiftly and was shown to be cost-effective without any extra purification. The proton NMR and infrared spectra were recorded. Characterization of the products was done by Proton NMR spectra in DMSO-d6 at 400 MHz using a Bruker Avance 400 spectrometer, with tetramethyl silane serving as the internal standard. TLCs were carried out using Merck TLC silica gel 60 F254 plates, which were eluted with EtOAc and developed with iodine.
Amla, the wonder berry has many nutraceutical, clinical and various functional properties. Ours is the first research study which employs AJE as a green and natural catalyst for the synthesis of acylated derivatives using microwave techniques which are brisk and free of lengthy procedures. AFE is less expensive than other catalysts, and the approach is environmentally beneficial. Equimolar acyl chlorides are employed without producing waste and provide simple experimental and workup operations. Under the experimental conditions, neither the aromatic ring nor a substituted group, such as the nitro group, is acylated (A2). N-acylation and O-acylation (A1-A7) occurred within 2 – 8 minutes under microwave irradiation using AFE as catalyst and their physical data are recorded in Table 1. The method has several advantages over the traditional ones mainly because of the absence of metal catalysts like FeCl3 or AlCl3.
There are reports in the literature for simple and ecologically friendly procedures for the synthesis of different aromatic derivatives with long reaction periods employing Cocos nucifera L. juice, Vitis vinifera L. juice, Solanum lycopersicum L. juice, and Citrus limetta juice 18-20. Like our studies, the use of MWs as an alternative energy source for catalytic reactions with shorter synthesis times was reported 24, 25. Acetylation is dramatically accelerated in micellar media cetyltrimethylammonium bromide, sodium dodecyl sulphate, and Triton‐X100 under reflux conditions 26, 27. We performed a kinetic investigation of silver nanoparticle production utilizing Phyllanthus emblica amla extract at different concentrations 28. AFE is a promising green and natural catalyst that has the potential to replace traditional catalysts in many organic synthesis reactions. It is a safe and effective catalyst that can be used to create a wide range of chemicals compounds but there are no reports on acetylation reactions employing AFE.
Table 1: Thin layer chromatography (Rf) Value, Yield, pH Value
|
S. No. |
Product |
Yield (%) |
Time (Minutes) |
Rf Values |
pH Values |
|
A1 |
Naphthalene-2-yl-acetate |
93% |
5 |
0.66 |
9 |
|
A2 |
N-(2-Nitrophenyl) acetamide |
96% |
3 |
0.46 |
6 |
|
A3 |
N-phenyl acetamide |
61% |
7 |
0.76 |
7 |
|
A4 |
1-phenylpropane-1,2-dione |
64% |
4 |
0.54 |
4.5 |
|
A5 |
Acetic benzoic anhydride |
95% |
2 |
0.77 |
4 |
|
A6 |
N-acetyl benzamide |
73% |
5 |
0.67 |
7 |
|
A7 |
Phenyl acetate |
85% |
8 |
0.69 |
6.5 |
Conclusion
When compared to other catalysts in the literature, Amla juice extract has the best catalytic activity in terms of product yield and reaction time. Our ability to further study other popular fruit juices as a green reaction medium for microwave-assisted reactions has been made possible by this work. Therefore, research into using other common fruit juices as a green reaction medium for the aforementioned reasons is now ongoing in our lab.
Acknowledgements
We express our gratitude to Anwarul Uloom College, Hyderabad, India, for their financial support and for providing the facilities for this research.
Conflict of Interest
The authors have no conflict of interest.
Funding Sources
There is no funding sources
References
- De la Hoz A., Loupy A. Microwaves in Organic Synthesis. 3rd Ed. Wiley-VCH Verlag GmbH & Co. KGaA; Weinheim, Germany: 2013.
CrossRef - Ravichandran S.; Karthikeyan E.; Microwave synthesis-a potential tool for green chemistry. Int. J. Chem. Tech. Res. 2011, 3, 466-70.
- Ali, M. M.; Sariah, S.; Tasneem; Rajanna, K. C.; Saiprakash, P. K. Ultrasonically accelerated Vilsmeier Haack cyclization and formylation reactions, Synthetic Communications. 2002, 32(9), 1351-1356.
CrossRef - Boruah, P.R.; Ali, A.A.; Saikia, B.; Sarma, D. A novel green protocol for ligan free Suzuki – Miyaura cross-coupling reactions in WEB at room temperature, Green Chem. 2015, 17, 1442–1445.
CrossRef - Sheldon, R.A. Catalysis: The key to waste minimization. Journal of chemical Technology & Biotechnology. 1997, 68 (4), 381-388.
CrossRef - Gabano, E.; Ravera, M. Microwave-Assisted Synthesis: Can Transition Metal Complexes Take Advantage of This “Green” Method? Molecules. 2022, 27(13), 4249. doi: 10.3390/molecules27134249.
CrossRef - Shalaby, M. A.; Fahim, A. M.; Rizk, S. A. Microwave-assisted synthesis, antioxidant activity, docking simulation, and DFT analysis of different heterocyclic compounds. Sci Rep. 2023, 13(1), 4999. doi: 10.1038/s41598-023-31995-w.
CrossRef - Thakuri, A.; Banerjee, M.; Chatterjee, A.; Microwave-assisted rapid and sustainable synthesis of unsymmetrical azo dyes by coupling of nitroarenes with aniline derivatives. iScience. 2022, 25(6), 104497. doi: 10.1016/j.isci.2022.104497.
CrossRef - Rajanna, K. C.; Ali, M. M.; Sariah, S.; Tasneem and Saiprakash, P. K. Vilsmeier Haack Acetylation in Micellar media: An efficient one-pot synthesis of 2-chloro-3-acetyl quinolines. Journal of Dispersion Science and Technology, 2004, 25(1), 17-21.
CrossRef - Santiago, C.; Jiménez-Aberasturi, X.; Leicea, E.; Lete, M. G.; Sotomayor, N.; Lete, E. Microwave-assisted palladium catalysed C–H acylation with aldehydes: synthesis and diversification of 3-acylthiophenes. Organic and Biomolecular Chemistry, 2022, 20(4), 852-861.
CrossRef - Choudhary, V. R.; Tillu, V. H.; Narkhede, V. S.; Borate, H. B.; Wakharkar, R. D.; Microwave assisted solvent-free synthesis of dihydropyrimidinones by Biginelli reaction over Si-MCM-41 supported FeCl3 catalyst. Catal Commun, 2003, 4, 449-53.
CrossRef - Misra, A. K.; Agnihotri, G.; Madhusudan, S. K. Microwave induced eco-friendly solvent-free Biginelli reaction catalyzed by calcium chloride. Indian J Chem, 2004, 43B, 2018-20.
CrossRef - Sharma, S.; Mishra, S.; Gupta, M.; Mishra, A. Microwave assisted one pot synthesis, Mass spectral analysis and DFT studies of 6-Substituted-3,4-dihydro-4-phenylpyrimidin-2(1H)-one. J Mater Environ Sci, 2014, 5, 1079-84.
- Tanay Pramanik, Poulami Maji, Microwave assisted green synthesis of pharmaceutically important dihydropyrimidinones in fruit juice medium, Int J Pharm Pharm Sci, 2015, 7(11), 376-379.
- Schanche, J. S. Microwave synthesis solutions from personal chemistry. Mol. Divers, 2003, 7, 291–298. doi: 10.1023/B:MODI.0000006866.38392.f7.
CrossRef - Pramanik, T.; Pathan, A. H. Exploring the utility of fruit juices as green medium for biginelli reaction. Res J Pharm Biol Chem Sci, 2014, 5, 444-449.
- Gulati, S.; Singh, R.; Prakash, R.; Sangwan, S. One-pot three component synthesis of substituted dihydropyrimidinones using fruit juices as biocatalyst and their biological studies. PLoS One. 2020, 15(9), e0238092. doi: 10.1371/journal.pone.0238092.
CrossRef - Gulati, S.; Singh, R.;, Sangwan, S. Fruit juice mediated multicomponent reaction for the synthesis of substituted isoxazoles and their in vitro bio-evaluation. Sci Rep, 2021, 11(1), 23563. doi: 10.1038/s41598-021-03057-6.
CrossRef - Gulati, S.; Singh, R.; Sangwan, S. Fruit juices act as biocatalysts in the efficient synthesis of potentially bioactive imidazoles, Green Chemistry Letters and Reviews, 2022, 15:1, 3-17. doi: 10.1080/17518253.2021.2013551.
CrossRef - Marvi, O.; Fekri, L. Z. Citrus Juice: Green and Natural Catalyst for the Solvent-free Silica Supported Synthesis of β-Enaminones Using Grindstone Technique. Comb Chem High Throughput Screen. 2018, 21(1), 19-25. doi: 10.2174/1386207321666180102115733.
CrossRef - Bagwan, S.M.; Farooqui, M.; Pathan, M. A.; Patil, S.M.; and Asif, M. Green Approach For The Preparation Of 2-Aryl-1, 3-Benzoxazole Derivatives By Using Amla Fruit Extract. Rasayan J. Chem., 2023,16(2), 699-706. http://doi.org/10.31788/RJC.2023.1628174.
CrossRef - Masum, M.M.I.; Siddiqa, M.M.; Ali, K.A.; Zhang, Y.; Abdallah, Y.; Ibrahim, E.; Qiu, W.; Yan, C.; Li, B. Biogenic Synthesis of Silver Nanoparticles Using Phyllanthus emblica Fruit Extract and Its Inhibitory Action Against the Pathogen Acidovorax oryzae Strain RS-2 of Rice Bacterial Brown Stripe. Front Microbiol. 2019, 10:820. doi: 10.3389/fmicb.2019.00820.
CrossRef - Mahato, A. K.; Sahoo, B. M.; Banik, B. K.; Mohanta, B. C. Microwave-assisted synthesis: Paradigm of green chemistry. J. Indian Chem. Soc. 2018, 95, 1327–1339.
- Díaz-Ortiz, Á.; Carrillo, J. R. Microwaves in green and sustainable chemistry. In: Cravotto G., Carnaroglio D., editors. Microwave Chemistry. De Gruyter; Berlin, Germany: 2017. pp. 167–183.
CrossRef - Mohammed. T.; Khan, A. A.; Iqubal, S. M. S.; Dawoud, A.; Khan, K. A.; Maqbul, M. S.; Fathima, S. H. A Kinetic and Synthetic approach of Surfactants accelerated by Vilsmeier-Haack acetylation and formylation with anisole, Asian J. Pharm. Sci., 2021, 15(3), 365-371, Doi: http://dx.doi.org/10.22377/ajp.v15i3.4152.
CrossRef - Mohammed. T.; Khan, A. A.; Iqubal, S. M. S.; Alyami, B. A. Micellar effects on kinetics and mechanism of Vilsmeier-Haack formylation and acetylation with Pyridines, Chem. Pap. 2022, https://doi.org/10.1007/s11696-022-02066-7.
CrossRef - Iqbal, N.; Iqubal, S. M.; Khan, A. B.; Mohammed, T.; Alshabi, A. M.; Aazam, E. S.; Rafiquee, M. Z. A. Effect of CTABr (surfactant) on the kinetics of formation of silver nanoparticles by Amla extract. Journal of Molecular Liquids, 2021, 329(2): 115537.
CrossRef

This work is licensed under a Creative Commons Attribution 4.0 International License.









