Polyethene Glycol (PEG-400): An efficient and Eco-Friendly Catalyst for the Preparation of N-benzylideneaniline by Schiff Base Reaction
1Department of Chemistry, Amity University, Jaipur, Rajasthan, India.
2Department of Chemistry, Sunrise University, Alwar, Rajasthan, India.
3Bhaskaracharya College of Applied Science, University of Delhi, Delhi, India.
4Faculty of Science, Amity University, Jaipur, Rajasthan, India.
Corresponding Author E-mail: bansalpalvi29@gmail.com
DOI : http://dx.doi.org/10.13005/ojc/380422
Article Received on : 13 Jul 2022
Article Accepted on :
Article Published : 18 Aug 2022
Reviewed by: Dr. Rafid Saad Dawood
Second Review by: Dr. Wayan Sutapa
Final Approval by: Dr.MGH Zaidi
Schiff base compounds are gaining importance daily in this scenario due to their various biological and catalytic activities. In this study, an efficient and eco-friendly synthesis of N-benzylideneaniline was carried out in polyethene glycol (PEG-400) as a greener medium at room temperature. PEG400 was inexpensive and non-toxic, providing a high yield and efficient medium for the synthesis of Schiff bases with excellent outcomes. Furthermore, FT-IR spectroscopy is used to characterize the newly created Schiff base. The recyclability of the catalyst was also studied up to six cycles.
KEYWORDS:Benzylideneaniline; Polyethene glycol (PEG400); Recyclable catalyst; Schiff base
Download this article as:| Copy the following to cite this article: Jindal P, Kumar P, Sharma U, Chauhan M. S. Polyethene Glycol (PEG-400): An efficient and Eco-Friendly Catalyst for the Preparation of N-benzylideneaniline by Schiff Base Reaction. Orient J Chem 2022;38(4). |
| Copy the following to cite this URL: Jindal P, Kumar P, Sharma U, Chauhan M. S. Polyethene Glycol (PEG-400): An efficient and Eco-Friendly Catalyst for the Preparation of N-benzylideneaniline by Schiff Base Reaction. Orient J Chem 2022;38(4). Available from: https://bit.ly/3CjBEeB |
Introduction
The study of Schiff base metal complexes has become more critical in chemistry. Schiff bases are the compounds containing the azomethine group (-HC=N-). Hugo Schiff initially described them in 1864 as the condensed products of aldehydes or ketones1. Schiff bases have a wide range of applications in biology, “Many of these include ant- tubercular, antibacterial, antifungal, antitumor, diuretic insecticidal, herbicidal, anthelmintic anti-HIV, anticonvulsant, antihypertensive, and antiparasitic” 2,3. Schiff’s base derivatives have been studied extensively and used in various applications for over a century, including magneto chemistry, nonlinear optics4, photophysical research5, catalysis, materials chemistry, chemical analysis, and oxygen absorption and transport6. A wide variety of Schiff base Zn(II) complexes have been shown to be useful in the synthesis of electroluminescent materials. “Oxidative cyclohexane to cyclohexanol reactions are catalyzed by the use of Ru(III), Fe(III), and Co(II) complexes of Schiff bases produced from hydroxybenzaldehyde7. A catalytic precursor for olefin oligomerization may be generated from cyclohexanone with H2O2 and Ni(II) complexes with bivalent (N-N) ligands”8. The dye and polymer industries are said to benefit from Schiff bases. It has been claimed that chromium and cobalt complexes of Schiff base may colour leather, food packaging and wool. Its nickel complex (Ni(II)-2L) was also synthesized and studied for its properties. “In the case of 5-diethylamino-2, the two most common phenolic compounds are 5-diethylamine-2 and 5-diethylamino-2-(dimethylamino)phenylamine-2. Along with their metal(II) complexes [Cu(L1)2](1), (2), (3), and (4) functioning as anticancer agents were synthesized as novel Schiff base ligands”9. Co/Ni/Cu/Zn(II) Synthetic Schiff base complexes formed from furfural-MAP and 6-methyl-2-aminopyridine were produced10. However, it was observed that longer reaction times, high temperatures, low yields, and the use of carcinogenic media are some of the drawbacks of the reported methods. Therefore, some efforts are made by chemists to modify the synthesis of Schiff bases by employing an efficient synthetic route using green tools.
In the last decade, solvents have increased significantly. To synthesize compounds in chemical processes, selecting an appropriate solvent and catalyst is essential. During the past few years, considerable attention has been received to various organic syntheses towards polyethene glycol (PEG) because it is non-toxic, cost-effective, readily available, nonflammable, nonvolatile and has high stability and has a safe character11. Moreover, PEG remains neutral in the system, due to which various functional groups remain undisturbed that is either acidic or basic. For these reasons, PEG is considered environmentally susceptible. Thus, has for different chemical transformations, PEG-400 emerged as an efficient catalyst. Furthermore, PEG-400 enhanced the reaction rate by forming strong hydrogen bonds with the substrate. As a result, the PEG-400 is widely used in many organic reactions to convert oxiranes to thiranes, asymmetric aldol condensation reactions, cross-coupling reactions, and reaction media for mono-bromination aromatics using NSB, synthesis of derivatives of triazole and pyrazole12. We have developed an efficient and environmentally benign synthesis of N-benzylideanilines using PEG400 as a catalyst, considering PEG’s synthetic utility13.
Experimental
Materials and Methods
Materials
No purification was performed on any chemical reagents. Benzaldehyde, aniline and its derivatives, hexane, diethyl ether, toluene, DCM, ethyl acetate, magnetic stirrer, PEG-400 etc. For characterization of compounds, TLC, Bruker FT-IR Spectrometer, and Bruker advance neo 500MHz NMR Spectrometer are used.
General Procedure
Sample Preparation
Preparation of Schiff base and their derivatives
Initially, benzaldehyde (1.0 mmol) is added to PEG-400 (10 μL), and then aniline (1.0 mmol) is added to the reaction. The reaction mixture was agitated for 45 minutes at room temperature using a magnetic stirrer. After that, the product is monitored by TLC. The desired outcome is crystallized by hexane and ethyl acetate into ice. The product’s solid form, i.e., Schiff base (N-benzylideaniline), is filtered and stored for characterization. The desired product was isolated in 98% yield. Similarly, following this method, we have done reactions between derivatives of aniline and Benzaldehyde.
Detection Method
FT-IR of PEG-400 and N-benzylideneaniline
The FT-IR spectra of fresh PEG-400 and recoverable PEG-400 were compared. Both show almost the same absorption peaks. When it comes to bending and stretching, polyethene glycol has you covered. In the 3446 cm-1 area, the OH stretch is turning14-16. It was a bending vibration of CH2, causing absorption of around 1483 cm-1. The C-O stretching vibration reveals a significant activity band at 1348 cm-1 to 1247 cm-1. C-C stretching leads to a sharp bands of 975 cm-1 and 868 cm-(1)18. The C=N extension of N-benzylideneaniline appears at 1624 cm-1, and the C=C stretching band seems at 1589 cm-1 (Figure 2)
 |
Figure 1: FTIR Spectrum of PEG-400 and N-Benzylideneaniline. |
NMR of N-Benzylideneaniline
Yellowish Solid, m.p. 51 ºC, yield: 98%, 1H NMR (δH, ppm): 8.28 (1H), 7.76 (2H), 7.75-7.29 (7H) and 7.10-7.07 (1H).
13C NMR (δC, ppm): 160.5, 152.2, 136.3, 131.5, 129.4, 126.1, 121.00, 118.6, 115.2
 |
Figure 2: 1H NMR spectrum of Schiff’s base |
 |
Figure 3: 13C NMR spectrum of Schiff’s base. |
Results and Discussion
In our model reaction, N-benzylideaniline is synthesized using benzaldehyde and aniline (Scheme 1). Such a reaction is catalyzed by polyethene glycol (10μl), providing a 98% yield in less than 1 hr of the relative compound under neat conditions (Table 2, Entry 1). The reaction of benzaldehyde with aniline was examined in different solvents such as hexane, diethyl ether, toluene, DCM, and PEG (400)19. The comparisons and results are shown in Table 1. The examination shows that in solvents like hexane, diethyl ether, toluene and DCM, the product yield varies from 65%, 60%, 70%, and 68%, respectively (Table 1). Among the tested solvents, it is concluded that the reaction in PEG-400 is more facile and proceeds to give a good yield (98%) at room temperature in neat conditions20.
 |
Scheme 1: Schiff’s base reaction using benzaldehyde and aniline. |
Table 1: Optimization for the synthesis of N-benzylideneaniline.
|
Entry |
Catalyst |
Solvents |
Reaction time (minutes) |
Yield(%) |
|
1 |
30 μL |
Hexane |
30 |
65 |
|
2 |
30 μL |
Diethyl ether |
30 |
60 |
|
3 |
30 μL |
Toluene |
30 |
70 |
|
4 |
30 μL |
DCM |
30 |
68 |
|
5 |
30 μL |
Neat |
30 |
85 |
|
6 |
20 μL |
Neat |
35 |
90 |
|
7 |
10 μL |
Neat |
40 |
98 |
|
8 |
Without Catalyst |
Neat |
60 |
35 |
To determine the full extent of the shift, the reaction of various derivatives of benzaldehyde with multiple products of aniline is carried out with optimized reaction conditions (Table 2). The effect of electron-withdrawing and electron-releasing substituents is also observed in the synthesis of N-benzylideaniline21. Aniline gives a higher yield of the relative compound with electron releasing derivatives like 4-Me, 4-OMe, and 4-OH with 95%, 80%, and 72% (Table 2, entries 2,3 and 4) yields of the relative compound, respectively. Simultaneously, electron-withdrawing groups like 4-NO2 and 4-Cl give 85% and 90% (Table 1, entries 5 and 6) of the comparative compound22.
 |
Scheme 2 Click here to View scheme |
Table 2: Preparation of N-benzylideneaniline and their derivatives by Schiff base reaction23 with PEG-400.
|
Entry |
R1 |
R2 |
Yield (%) |
|
|
1 |
H |
H |
98 |
|
|
2 |
H |
4-OH |
72 |
|
|
3 |
H |
4-CH3 |
95 |
|
|
4 |
H |
4-OCH3 |
80 |
|
|
5 |
H |
4-NO2 |
85 |
|
|
6 |
H |
4-Cl |
90 |
|
|
7 |
4-OH |
H |
85 |
|
|
8 |
4-OH |
4-OH |
75 |
|
|
9 |
4-OH |
4-CH3 |
95 |
|
|
10 |
4-OH |
4-OCH3 |
75 |
|
|
11 |
4-OH |
4-NO2 |
90 |
|
|
12 |
4-OH |
4-Cl |
70 |
|
|
13 |
4-Br |
H |
98 |
|
|
14 |
4-Br |
4-OH |
80 |
|
|
15 |
4-Br |
4-CH3 |
85 |
|
|
16 |
4-Br |
4-OCH3 |
65 |
|
|
17 |
4-Br |
4-NO2 |
78 |
|
|
18 |
4-Br |
4-Cl |
70 |
|
|
19 |
4-NO2 |
H |
85 |
|
|
20 |
4-NO2 |
4-OH |
60 |
|
|
21 |
4-NO2 |
4-CH3 |
80 |
|
|
22 |
4-NO2 |
4-OCH3 |
90 |
|
|
23 |
4-NO2 |
4-NO2 |
60 |
|
|
24 |
4-NO2 |
4-Cl |
95 |
|
Recyclability test: The PEG-400 in the reaction mixture was recovered24 and reused with no loss of activity. The recyclability of the PEG-400 was seven times greater than previously thought (Figure 1).
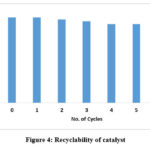 |
Figure 4: Recyclability of catalyst. |
Conclusions
Schiff base is used as an active pharmaceutical agent25. A Schiff base reaction was synthesized using benzaldehyde and aniline with an excellent yield (98%). PEG-400 catalyzed the response. The catalyst can be recyclable. The derivatives of the Schiff base reaction were also synthesized. The desired product is confirmed using FT-IR and NMR spectroscopy.
Acknowledgements
The authors convey gratitude to AMITY University JAIPUR, Rajasthan (India) and SUNRISE University, Alwar, Rajasthan (India), and Dr M.S.Chauhan Sir for their guidance and support in providing instrumental facilities for the research work.
Conflicts of Interest
The authors declare no conflicts of interest.
Funding Sources
There is no funding source.
References
- D. A. Xavier and N. Srividhya, IOSR Journal of Applied Chemistry. 2014, 7, 06–15
CrossRef - E. Ergun, Ü. Ergun, Ö. İleri and M. F. Küçükmüzevir, Spectrochimica Acta – Part A: Molecular and Biomolecular Spectroscopy, 2018, 203, 273–286
CrossRef - C. Valverde, Í. N. Ribeiro, J. V. B. Soares, B. Baseia and F. A. P. Osório, Advances in Condensed Matter Physics, DOI:10.1155/2019/8148392.
CrossRef - P. G. Cozzi, L. S. Dolci, A. Garelli, M. Montalti, L. Prodi and N. Zaccheroni, New Journal of Chemistry, 2003, 27, 692–697
CrossRef - M.K.Mishra, Rasayan J. Chem, 2022, 15(1), 217-220; http://dx.doi.org/10.31788/RJC.2022.1516206
CrossRef - N. Pal, M. Pramanik, A. Bhaumik and M. Ali, Journal of Molecular Catalysis A: Chemical, 2014, 392, 299–307
CrossRef - F. Speiser, P. Braunstein and L. Saussine, Organometallics, 2004, 203,2625–2632
CrossRef - C. Vidya Rani, M. P. Kesavan, S. Haseena, R. Varatharaj, J. Rajesh and G. Rajagopal, Applied Biochemistry and Biotechnology, 2020, 191 1515–1532.
CrossRef - L. John, R. S. Josephus and I. H. Joe, SN Applied Sciences, DOI:10.1007/s42452-020-2274-6.
CrossRef - R. S. Mekala, S. K. Balam, J. P. S. Harinath, R. R. Gajjal and S. R. Cirandur, Cogent Chemistry, 2015, 1, 1049932
CrossRef - M. Shaikh, D. Wagar, M. Farooqui and A. Durrani, Polycyclic Aromatic Compounds, 2020, 40, 1315–1320 DOI: 10.2174/1570179417666200529121602
CrossRef - L.G. Buttke, J.R. Schueller, C.S. Pearson and K.D. Beyer, J. Phys. Chem. A, 2016, 120, 6424 ; https://doi.or g/10.1021/acs.jpca.6b05208
CrossRef - P. Jindal and M. S. Chauhan, Rasayan J. Chem, 2022, 15 (1), 538-548; http://dx.doi.org/10.31788/RJC.2022.1516748
CrossRef - P. Kumar and P. Gupta, Oriental Journal of Chemistry, 2021, 37 (3), 594-601; http://dx.doi.org/10.13005/ojc/37031
CrossRef - B.T. Matsuo, P.H.R. Oliveira, J.T.M. Correia and M.W. Paixão, Org. Lett., 2021, 23, 6775; https://doi.or g/10.1021/acs.or glett.1c02353
CrossRef - B. Singh, J. S. Aulakh and B. Singh, Asian J. Chem., 2022, 34(6), 1549-1554 https://doi.org/10.14233/ajchem.2022.23715
CrossRef - A. W. Walke and N. E. Kathle, Asian J. Chem., 2022, 34(6), 1488-1492 https://doi.org/10.14233/ajchem.2022.23717
CrossRef - N. Anthil, M. Kumar, K.K.Verma and S.Garg, Asian J. Chem., 2022, 34(5), 1125-1133; https://doi.org/10.14233/ajchem.2022.23615
CrossRef - N.V. Junghare, S. B. Jagtap, R. R. Jhadav and J.P.Jhadav, Asian J. Chem.,2022, 34(3), 657-662; https://doi.org/10.14233/ajchem.2022.23572
CrossRef - S.S.Kotha, K.V.Mohan, R. Doddipalla et. Rasayan J. Chem, 2022, 15 (1), 82-95 http://dx.doi.org/10.31788/RJC.2022.1516527
CrossRef - N.G. Devi, N.V. Rao, D. Ramchandaran and P.S. Rani, Rasayan J. Chem, 2022, 15 (1), 292- 301 http://dx.doi.org/10.31788/RJC.2022.1516663.
CrossRef - Mishra, K.U.; Siddiqi, A.; Meikap, H, J. Cleon. Prod. 2021, 279,123645
CrossRef - Z. Li, Y. Du, H. Lu, A. Yang and J. Yang, Green Process. Synth., 2019, 8, 93; https://doi.or g/10.1515/gps-2018-0017
- X. Zhang, A. Xia, H. Chen and Y. Liu, Org. Lett., 2017, 19, 2118; https://doi.org/10.1021/acs.or glett.7b00732
CrossRef

This work is licensed under a Creative Commons Attribution 4.0 International License.










