Studies on Oxidation of Alcohols by Cerium (IV)-Dimer in Aqueous Nitric Acid
Bhuwan Chandra1*, Anshu Tamta1, Rashmi Khulbe1, Asha Kandpal1, N.D. Kandpal1 and Kailash Tamta2
1Physical Chemistry Laboratory, Department of Chemistry, Soban Singh Jeena University, Campus, Almora-263601, Uttarakhand, India.
2Government Degree College Kanda, Bageshwar-263619, Uttarakhand, India.
Corresponding Author E-mail: bc_physical@rediffmail.com
DOI : http://dx.doi.org/10.13005/ojc/380116
Article Received on : 29-Oct-2021
Article Accepted on : 04-Jan-2022
Article Published : 08 Jan 2022
Reviewed by: Dr. Falah Kareem Hadi
Second Review by: Dr. Bhuwan Chandra
Final Approval by: Dr. Nenad Ignjatovic
The oxidation of alcohols, n-propanol, 2-methoxy ethanol and 2-ethoxy ethanol by cerium (IV) in nitric acid leads to the formation of corresponding aldehydes. The reaction is first order with respect to Ce(IV). A Michaelis-Menten type kinetics is observed with respect to alcohols. The reaction is retarded by initial [Ce(IV)] with increase in concentration of oxidant. The reaction exhibits participation of 2 mole of cerium (IV) with 1 mole of alcohols. It has been concluded that Ce(IV)-dimer participate in the oxidation process and formation of free radical through complex ion formation in the nitric acid medium.
KEYWORDS:Alcohols; Cerium (IV) Oxidant, Ce2(NO3)6++; Redox kinetics
Download this article as:| Copy the following to cite this article: Chandra B, Tamta A, Khulbe R, Kandpal A, Kandpal N.D, Tamta K. Studies on Oxidation of Alcohols by Cerium (IV)-Dimer in Aqueous Nitric Acid. Orient J Chem 2022;38(1). |
| Copy the following to cite this URL: Chandra B, Tamta A, Khulbe R, Kandpal A, Kandpal N.D, Tamta K. Studies on Oxidation of Alcohols by Cerium (IV)-Dimer in Aqueous Nitric Acid. Orient J Chem 2022;38(1). Available from: https://bit.ly/3G7V59f |
Introduction
Kinetics and mechanistic studies on oxidation of various organic substrates by cerium salts have been reported in perchloric acid1,2,3,4, sulphuric acid5,6,7,8 and nitric acid9,10. Selective oxidation of substrates in the aqueous solution is significant in order to investigate the proper and efficient reactants and their species like oxidants, catalysts activity of the different solvent systems. Cerium (IV) ion has been used for oxidation of many organic compounds in the form of ceric ammonium nitrate and ceric ammonium sulphate. The resulting species depends upon the nature of the acid present in the reaction media. There are reports on oxidation of alcohols with complex mechanism in which disproportionation of complex yields cerrous ion, a proton and free radical on the alcohol substrate11,12. It has been established that cerium (IV) forms dimers and polymers in perchloric acid13. It had been reported that kobs decreased with the increasing cerium (IV) concentration in the oxidation of acetone14, glycine15, carboxylic acids16,17 and phenethyl alcohol and para-substituted phenethyl alcohols18 in the nitric acid medium. The present study represent kinetics and mechanism of oxidation of alcohols, n-propanol, 2-methoxy ethanol and 2-ethoxy ethanol by cerium(IV) in nitric acid medium in order to ascertain the participation of active cerium(IV) –dimeric species of oxidant in the mechanistic pathways.
Experimental
The reaction were studied under pseudo first order condition by keeping [alcohol] >> [Ce(IV)]. The reaction were studied at constant temperature (±0.1K) and followed by monitoring the decrease in the [Ce(IV)] volumetrically. The reaction mixtures quenched in the same volume of Fe(II) solution which was titrated against cerium IV sulphate solution of the same strength as that of Fe(II) solution, using nitro ferroin as indicator. The micro burette reading therefore directly gave the value of cerium (IV) used in the reaction.
Evaluation of Rate Constant
The value of (-dc/dt) were calculated from the plots between remaining ceric ammonium nitrate and time. The value of kobs was calculated by dividing (-dc/dt ) value of concentration of [cerium (IV)].
Stoichiometry
The stoichiometry in the oxidation of alcohols was determine under the experimental condition to those similar condition of the kinetic study [Alcohol] >>[Ce (IV)].Under the kinetic conditions the product aldehydes were identified in case of alcohols which are estimated gravimetrically as 2,4–dinitrophenylhydrozone. The solution of 2,4-dinitrophenylhydrozone was prepared as described in the literatute19. For four independent determination ,the ratio of │∆[cerium(IV)]T │/│∆[acetaldehyde]T was found to be 2/1. It indicates that one mole of alcohol consumes two mole of cerium(IV),the following stoichiometric equation is proposed.
RCH2OH + 2Ce (IV) → RCHO +2Ce(III) +2H+
Test for Free Radical
The reaction mixture was degassed with nitrogen before the reaction was initiated. The addition of acrylonitrile is partially oxidized in case of each alcohol in mixtures. The formation of viscous/curdy type precipitate indicated the presence of free radical in the reaction process.
Dependence of Rate on Initial Cerium (IV) Concentration
Under the condition [alcohol]>> [Ce(IV)] in nitric acid media the rate of disappearance of cerium (IV) shows a first order dependence on cerium(IV) concentration. If initial concentration of cerium (IV) is [Ce (IV)]0 the dependence is given by
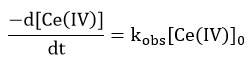
The equation indicates that kobs should be independent of initial Cerium (IV) concentration, which is one of the characteristic of first order reaction. But in this study kobs decreased with increasing [Ce(IV)] in the case of each alcohol. The rate data are given in Table 1 and the linear plots between [Ce(IV)]-1 and kobs are shown in Figure 1, 2 and 3.
Table 1: Dependence of kobs on Initial Cerium (IV) Concentration at 298 K
[H+] = 0.575 mol dm-3; [Alcohol] = 0.04 mol dm-3
|
10³ [Ce(IV)] |
kobs (s-1)× 104 |
||
|
n-propanol |
2-methoxy ethanol |
2-ethoxy ethanol |
|
|
1.4 |
2.68 |
1.42 |
0.80 |
|
2.1 |
1.92 |
0.98 |
0.59 |
|
2.8 |
1.54 |
0.79 |
0.47 |
|
4.2 |
1.08 |
0.57 |
0.34 |
|
5.6 |
0.84 |
0.44 |
0.29 |
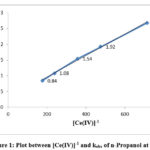 |
Figure 1: Plot between [Ce(IV)]-1 and kobs of n-Propanol at 298K |
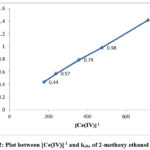 |
Figure 2: Plot between [Ce(IV)]-1 and kobs of 2-methoxy ethanol at 298K |
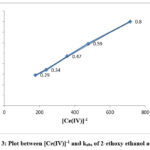 |
Figure 3: Plot between [Ce(IV)]-1 and kobs of 2-ethoxy ethanol at 298K |
Dependence of Rate on Initial Substrate Concentration
The effect of varying the initial alcohol was investigated over a range 0.04-0.20 mol dm-3 of alcohols. The values of rates increases with increasing initial concentration of the substrate for each alcohol in solution 0.5moldm-3 nitric acid. The variations of kobs values for n-propanol, 2-methoxy ethanol and 2-ethoxy ethanol are given in Table 2. The plot between kobs-1 and [alcohol]-1 followed the Michaelis-Menten type correlation. The representative plots are given Figure 4, 5 and 6.
Table 2: Dependence of kobs on Initial Alcohol Concentration
103[Ce(IV)] = 1.40 mol dm-3; [H+] = 0.50 mol dm-3; [NO3–] = 0.50 mol dm-3
|
[n-propanol ] mol dm-3
|
kobs(s-1)× 103 |
|||
|
298K |
303K |
307K |
311K |
|
|
0.04 |
0.30 |
0.65 |
1.04 |
1.50 |
|
s0.08 |
0.53 |
1.13 |
1.88 |
2.67 |
|
0.12 |
0.68 |
1.50 |
2.58 |
3.59 |
|
0.16 |
0.79 |
1.58 |
2.94 |
4.85 |
|
0.20 |
0.99 |
2.26 |
3.37 |
5.38 |
|
[2-Methoxy ethanol ] mol dm-3 |
kobs(s-1) × 103 |
|||
|
298K |
303K |
307K |
311K |
|
|
0.04 |
0.21 |
0.40 |
0.62 |
0.96 |
|
0.08 |
0.35 |
0.60 |
1.11 |
1.53 |
|
0.12 |
0.48 |
0.82 |
1.52 |
2.52 |
|
0.16 |
0.62 |
1.15 |
1.90 |
2.91 |
|
0.20 |
0.75 |
1.40 |
1.96 |
3.93 |
|
[2-Ethoxy ethanol ] mol dm-3 |
kobs(s-1) × 103 |
|||
|
298K |
303K |
307K |
311K |
|
|
0.04 |
0.39 |
0.50 |
0.78 |
1.04 |
|
0.08 |
0.65 |
0.85 |
1.33 |
1.92 |
|
0.12 |
0.87 |
1.25 |
1.95 |
2.62 |
|
0.16 |
1.12 |
1.58 |
2.42 |
3.00 |
|
0.20 |
1.17 |
1.66 |
2.54 |
3.44 |
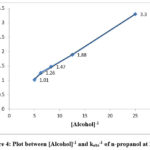 |
Figure 4: Plot between [Alcohol]-1 and kobs-1 of n-propanol at 298 K |
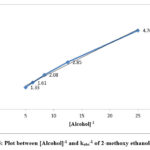 |
Figure 5: Plot between [Alcohol]-1 and kobs-1 of 2-methoxy ethanol at 298K |
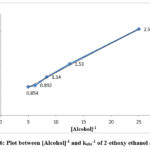 |
Figure 6: Plot between [Alcohol]-1 and kobs-1 of 2-ethoxy ethanol at 298K |
Dependence of Rate on Initial Nitric Acid Concentration
The kobs value was measured at different initial nitric acid concentration at constant concentration of nitrate ion with n-propanol, 2-methoxy ethanol and 2-ethoxy ethanol. The results are given in Table 3. It was noted that in each case kobs decreased with increasing [nitric acid]0.
Table 3: Dependence of kobs on Initial Nitric Acid Concentration at 298 K
103[Ce(IV)] = 1.40 mol dm-3; [Alcohol] = 0.04 mol dm-3;[NO3–] = 1.20 mol dm-3
|
[H+] |
Kobs (s-1)×104 |
||
|
n-propanol |
2-methoxy ethanol
|
2-ethoxyethanol
|
|
|
0.4 |
2.60 |
1.04 |
1.88 |
|
0.6 |
2.52 |
0.90 |
1.70 |
|
0.8 |
2.42 |
0.80 |
1.52 |
|
1.0 |
2.30 |
0.72 |
1.34 |
|
1.2 |
2.18 |
0.61 |
1.20 |
Dependence of Rate on Initial Nitrate Ion Concentration
In aqueous nitric acid solution cerium (IV) forms Ce(IV)-nitrate complexes. Hence it was felt useful to investigate the effect of initial [NO3–] on kobs. The investigations were carried out over the range of concentration 0.4 – 1.2 mol dm-3 at constant [nitrate ion]. The results obtained in the case of different alcohols are collected in Table 4.
Table 4: Dependence of kobs on The Initial Nitrate Ion Concentration at 298 K
103[Ce(IV)] = 1.40 mol dm-3; [Alcohol] = 0.04 mol dm-3; [H+] = 0.50 mol dm-3
|
[NaNO3]
|
[NO3–] |
Kobs (s-1)×104 |
||
|
n-propanol |
2-methoxy ethanol
|
2-ethoxyethanol
|
||
|
0.4 |
0.9 |
3.21 |
2.20 |
3.32 |
|
0.6 |
1.1 |
3.01 |
2.02 |
3.17 |
|
0.8 |
1.3 |
2.85 |
1.92 |
3.00 |
|
1.0 |
1.5 |
2.70 |
1.80 |
2.83 |
|
1.2 |
1.7 |
2.50 |
1.70 |
2.70 |
Dependence of Rate on Initial Ce (III) of Concentration
The progress of the reaction followed first order rate reaction which indicated that the product form during the reaction did not affect the rate.This fact was also confirmed by the initial addition of cerium(III) in the reaction mixture. The results are given in Table 5. These results indicated that kobs were independent of Ce(III).
Table 5: Dependence of kobs on Initial Ce(III) Concentration at 298 K
103[Ce(III)] = 1.40 mol dm-3; [Alcohol] = 0.04 mol dm-3; [H+] = 0.50 mol dm-3
|
103[Ce(III)] = 1.40 mol dm-3
|
kobs (s-1) ×104 |
||
|
n-propanol |
2-methoxy ethanol |
2-ethoxy ethanol |
|
|
0.4 |
3.00 |
2.16 |
3.30 |
|
0.6 |
3.02 |
2.18 |
3.32 |
|
0.8 |
3.06 |
2.14 |
3.28 |
|
1.0 |
3.06 |
2.10 |
3.28 |
|
1.2 |
3.04 |
2.12 |
3.30 |
Mechanism and Discussion
In the oxidation by ceric ammonium nitrate in nitric acid media has been reported that the increase [NO3–] resulted decrease in the rate of oxidation20.A survey of literature showed that the cerium(IV) was found partially in the form of dimer in acidic medium21.
The presence of dimeric cerium (IV) is indicated in our system by the kinetic results in the form of decrease in kobs with increasing Ce(IV) concentration. The ceric ion exists as Ce4+, (Ce[OH]3)3+ and [Ce-O-Ce]+6 in aqueous solution.The concentration of these species was found to vary with the concentration of nitric acid 22.
The presence of cerium (IV)-dimer in the nitric acid media was also confirmed in the oxidation of diols. In these studies it was interesting that the disappearance of cerium (IV) at given concentration always followed first order kinetics in each of the above reactions. Such peculiar behaviour of oxidation processes in the oxidation of diols in nitric acid indicatethe participation of cerium (IV) – dimer23.
The data gives a linear plot between 1/kobsand 1/alcohol with a definite intercept on the ordinate axis suggesting the rate expression.
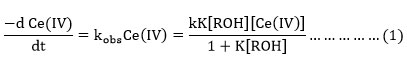
In our study the inverse of experimental rate constant versus the inverse of alcohol concentration has linear relation.The formation of the complex is similar to the mechanism proposed by previous work in the oxidation of primary and secondary alcohols 24. Based on the knowledge about the existence of various species of oxidant and reductant in nitrate medium and the results obtained in the study, the probable mechanism for the oxidation of alcohols could be given as follows.
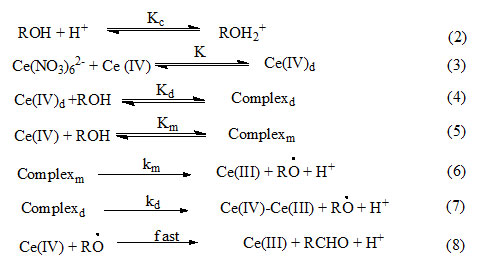
Where RÒ is free radical, the formation of which is tested by polymerization of acrylonitrile. In the redox system oxidant forms initially a complex by reacting with organic molecules which then decomposes to producefree radical that initiate polymerization25. The rate of disappearance of cerium (IV) in terms of reactions (2) – (8) is given by equation (9).
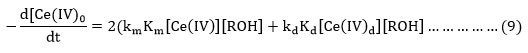
The total [cerium (IV)] at any instant is given by equation (10) where [Ce(NO3)2-] is represented by [CAN].
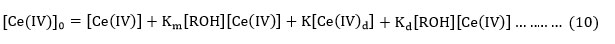
Substituting the value of [Ce(IV)]0from equation (10) in equation (9) , the rate equation (11) is derived.
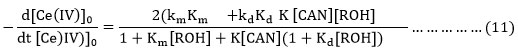
Now it is assumed as a major of approximation that K[CAN] (1+Kd [ROH]>1+Km[ROH]) and Kd is expected to be > Km, the equation (11) is reduced to equation (12).
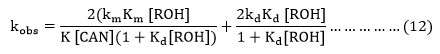
Where [ROH] = ROH]0/ 1+Kc[H+] and [ROH]0= Initial alcohol concentration.
The equation (12) is consistent with the linear plot between kobs and [Ce(IV)]-1 because the [CAN] is depends upon the initial cerium(IV) concentration.
Secondly the results obtained for each alcohol with the variation of nitrate ion the decrease in the rate with increasing initial nitrate ion concentration is consistent with the equation number (12), in which [CAN] includes the concentration of nitrate ion in the form of Ce2(NO3)62-.
Table 6: The value of slope and intercept of the plots kobs and [Ce(IV)]-1 at 298 K
[ROH]0= 0.04 mol dm-3; [H+] = 0.50 mol dm-3; [NO3–] = 0.50 mol dm-3 ; 103 [Ce(IV)] = 1.40 mol dm-3
|
Alcohol |
106 slope mol dm-3 sec-1 |
104 intercept sec-1 |
102 mol dm-3 |
|
n-propanol |
3.30 |
2.00 |
1.65 |
|
2-methoxy ethanol |
2.00 |
1.20 |
1.66 |
|
2-ethoxy ethanol |
1.00 |
0.80 |
1.25 |
It is clear from the data obtained in Table 6, the
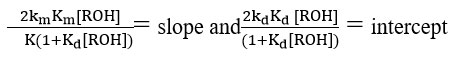
and
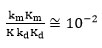
Confirmed the assumption that kmKm<<kdKd.
Hence for at any constant [CAN] the equation (12) can be approximated to equation (13).
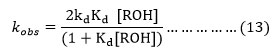
Again from equation (2),
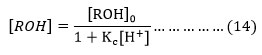
The substitution of the respective value of [ROH] in (13) gives the equation (15),
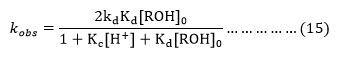
Equation (15) is the rate law in agreement with the equation (1) which is in agreement with the first order dependence in cerium (IV) for any initial ceium(IV) concentration. The equation (15) can be rearranged as,
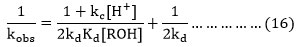
It is clear from equation (16) at constant nitrate ion concentration in the rate constant with increasing initial nitric acid concentration as obtained in case of each alcohol in our study is consistent with the rate expression given by equation (16). The plot between kobs-1 and concentration of H+ ions indicated a linear correlation.
From equation (16) it is apparent that (intercept/slope) of the plot between and (1/kobs and 1/alcohol) which are consistent with the (17) can be given as below.
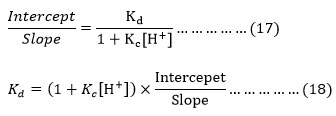
Table 7: Value of intercept and slope at different temperatures for the plots kobs-1 versus [Alcohol]-1
[Alcohol] = 0.04 mol dm-3; [H+] = 0.50 mol dm-3; [NO3–] = 0.50 mol dm-3; 103 [Ce(IV)] = 1.40 mol dm-3
|
Alcohol |
Temp. |
Slope |
Intercept |
|
|
n-propanol |
298K |
113.0 |
530.0 |
4.6 |
|
303K |
52.3 |
236.8 |
4.5 |
|
|
307K |
33.4 |
117.3 |
3.5 |
|
|
311K |
24.2 |
66.3 |
2.7 |
|
|
2-methoxy ethanol |
298K |
168.0 |
705.0 |
4.2 |
|
303K |
82.5 |
445.0 |
5.3 |
|
|
307K |
56.7 |
195.2 |
3.4 |
|
|
311K |
39.0 |
87.5 |
2.2 |
|
|
2-ethoxy ethanol |
298K |
110.3 |
240.0 |
2.2 |
|
303K |
71.9 |
222.5 |
3.0 |
|
|
307K |
45.8 |
148.5 |
3.2 |
|
|
311K |
33.7 |
113.0 |
3.3 |
The value of intercept and slope obtained from the plots between (1/[alcohol] and 1/kobs. For each alcohol at different temperatures are collected in Table 7. These values ≅Kd because low value [H+], [H+]×Kc<<1.
The intercept of the plots between 1/kobs and (1/[alcohol] and at different temperatures may be used for calculating the value of kd according to equation (16).
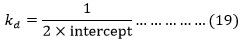
Table 8: Values of kd for each alcohol
|
Temperature |
103 kd (s-1) values |
|||
|
n-propanol |
2-methoxy ethanol |
2-ethoxy ethanol |
||
|
298K |
0.94 |
0.70 |
2.08 |
|
|
303K |
2.10 |
2.24 |
2.24 |
|
|
307K |
4.26 |
2.56 |
3.36 |
|
|
311K |
7.54 |
5.71 |
4.42 |
|
|
Enthalpy (Kcal) |
16 |
13 |
5 |
|
|
Entropy (cal deg-1 mol-1) |
-51 |
-52 |
-53 |
|
The value of kd obtained at each temperature are reported in Table 8.
The value of the kd reported in Table 8 were used for calculating the activation enthalpy and entropy. The value obtained for each alcohol is given in the bottom column of the table. These values are calculated by the linear plots between log kd against 1/T.
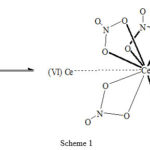
Finally considering the well-established fact that first order rate constant decreases with increasing concentration of Ce(IV), such behaviour account for the participation of polymeric and dimeric forms of Cerium (IV) 26.Thus from the complexing nature of Ce(IV) in nitric acid medium, it is reasonable that the Ce(IV) ions coordinated with the nitrate ions in bidentate fashion as shown below are involved in the oxidation process.
 |
Scheme 1 |
The above Ce(IV)-dimer has a resonating structure among different nitrate complexes.
Ce4+ — Ce (NO3)62-
(NO3)Ce3+ — Ce(NO3)5–
(NO3)2Ce2+ — Ce (NO3)4
(NO3)3Ce+ — Ce+ (NO3)3
We have to bear in mind that the whole structures is not fixed unit in solution , for a Ce(IV) can transfer rapidly from one Ce(NO3)62- structure to another because in aqueous solution the ion pair formation is less significant at low concentration.
The nitrate ion NO3– have three symmetrical canonical forms in which each oxygen atom has a change of 1/3 units. The greater the number of or double bonded ‘O’ atoms the greater is the delocalization of charge and greater is the acid strength in comparison to H2O molecule. In addition of formal charge and resonance possibilities, solvation of keto oxygen atom is also an acid strengthening whereas the solvation of the hydroxyl group is acid weakening, due to inductive effect of the water molecule in aqueous solution.
Hence Ce(IV) ion is expected to be in the form of complex nitrate ion rather than form aqua cation. [Ce(H2O)8]4+ or hydrolyzed Ce(OH)3+ ion in nitrate medium.
Acknowledgement
The authors are thankful to Dr. Raj. N. Mehrotra former professor and head, department of Chemistry, University of Jodhpur for helpful discussions and constant encouragement. Thanks are also to Dr. K.N Mathpal former head, department of Chemistry, Kumaun University, S.S.J. Campus Almora for providing lab facilities.
Conflicts of Interest
The authors declare no conflict of interest in the present research work.
Funding Sources
There are no funding source.
References
- Kandpal, N.D.; Joshi, S.K.; Mishra, V.N. Acta Chimica Hungarica. 1992,129(5),685-695.
- Naik, D.V.; Bayadagi, K.S.; Nanibewoor, S.T.; Chamatadar, S.A. Montash Chem. 2021,144,1307.
CrossRef - Fawzy, A.; Zaafarany, I.A.; Trikistani, F.A.; Al Bonayan, A.; Aliferry, F.A. Am J Phys Chem. 2016,5,10.
- Xu, L.; Tian, H.; Shri, T. Int J Chem Kinet. 2018,50,856.
CrossRef - Padhy, R.K.; Sahu, S. J Chem Sci. 2021,133(66),1-11.
CrossRef - Manjunath, D.M.; Pratibha, K.H; Patgar, T.N.; Gaudar, S.C.; Nandibewoor, S.T.; Chimatadar, S.A. Cogent Chem. 2016,2,1195243.
CrossRef - Tandon, P.K.; Khanam, S.Z.; Singh, S.B. The Open Catalysis J. 2012,5,1-7.
- Ghosh , A.; Saha, R.; Saha, B. J of molecular liquids. 2014,196,223-237.
CrossRef - Yagci, C.; Yildiz, U. Euro Polym J. 2005,41,177-184.
CrossRef - Joshi, S.R.; Kataria, K.L.; Sawant, S.B.; Joshi, J.B. Ind Eng Chem Res. 2005,44,325-333.
CrossRef - Arslan, H.; Hazer, B. Euro Polym J. 1999,35,1451.
CrossRef - Arslan, H.; Eroglu, M.S.; Hazer, B. Euro Polym J. 2001,37,581.
CrossRef - Yadav, M.B.; Vijay, D.; Ashu, R. J Indian Chem Soc. 2009, 86,600.
- Chandra, B.; Pandey, K.; Kandpal, N.D. J of Chemistry and Chemsciences. 2018,8(1),48-54.
CrossRef - Chandra, B.; Joshi, K.; Kandpal, N.D. International J of Scientific Research in Science Engineering and Technology. 2018,4(4),1384-1388.
- Nagori, R.R.; Mehta, M.; Mehrotra, R.N. Inorg Nucl Chem. 1981,43,2899-2904.
CrossRef - Kandpal, N.D.; Nagori, R.R.; Mehrotra, R.N. Can J Chem. 1986,64,19-23.
CrossRef - Rao, N.V.B.; Rao, M.A. Ind J Chem. 2005,44A,80-84.
CrossRef - Vogel, A.I. Elmentary Org Practical Chem Part-2 E.L.B.S Edn London. 1975,118.
- Rao, N.V.B.; Rao, M.A. Oxidation Commun. 2003,26(4),580.
CrossRef - Fawzy, A. J. Solution Chem. 2016,45,245-264.
CrossRef - Sharma, B.R.; Kumar, V.; Soni, P.L. J Applied Poly Sci. 2003,90,129-136.
CrossRef - Nagori, R.R.; Mehta, M.; Mehrotra, R.N. Ind J Chem. 1982,21A,41-47.
- Mata-Perez, F.; Francisco, C.; Alvarez, M.P. ZAnorgAllg Chem. 2002,628,431-436.
CrossRef - Yadav, M.B.; Devra, V.E.; Rani,A. Ind J Chem. 2010,49A,442-447.

This work is licensed under a Creative Commons Attribution 4.0 International License.









