Synthesis and Characterization of about 20nm Gold Nanoparticles
Department of Chemistry, Shibpur Dinobundhoo Institution (College), 412/1,G.T. Road (South), Howrah-711102, West Bengal, India.
Corresponding Author E-mail: abiswas83@gmail.com
DOI : http://dx.doi.org/10.13005/ojc/370524
Article Received on : 28-Jun-2021
Article Accepted on :
Article Published : 26 Oct 2021
Reviewed by: Dr. Subramaniam N.P

Second Review by: Dr M. Bala chander

Final Approval by: Dr. Balkrishna
The synthesis of spherical gold nanoparticles (AuNPs) by the chemical reduction process and the characterization of the synthesized nanoparticles is the main aim of this article. Reduction of Chloroauric acid by trisodium citrate salt was performed to get AuNPs of average diameter 20nm. Trisodium citrate is not only the reducing reagent but also the stabilizer of the synthesized AuNPs. Some important modern techniques like UV-VIS spectroscopy, diffraction light scattering (DLS), X-ray diffraction (XRD), transmission electron microscopy (TEM), Selected area electron diffraction (SAED) and electron diffraction X-ray (EDX) were involved for the characterization of synthesized AuNPs. Chemical reduction and Size-controlled growth of spherical AuNPs were followed for this particular synthesis of AuNPs.
KEYWORDS:AuNPs; EDX; SAED; TEM; UV; XRD
Download this article as:| Copy the following to cite this article: Biswas A. Synthesis and Characterization of about 20nm Gold Nanoparticles. Orient J Chem 2021;37(5). |
| Copy the following to cite this URL: Biswas A. Synthesis and Characterization of about 20nm Gold Nanoparticles. Orient J Chem 2021;37(5). Available from: https://bit.ly/3nt9Hrp |
Introduction
Colloidal nanoparticles (specifically metals) are becoming more and more significant in an assortment of scientific and technical fields. The gold nanoparticles (AuNPs) are perhaps the most significant members of the metal nanoparticles groups among all the metal colloidal nanoparticles. AuNPs are mostly studied and used nanoparticles over last 30 years. The applications of AuNPs are vast in the numerous field of catalysis, nanomedicines, microelectronics, optics and biotechnology.1-6 “Top down” and “bottom up” are the two usual procedure for the synthesis of AuNPs. The use of the first process is limited because it is difficult to control the shape and size of the AuNPs.7 The “bottom up” process is the wet chemical process of reduction of Au salts into AuNPs. The chemical reduction is simple and has a control over the size and shape in the synthesis of AuNPs.8 Spherical AuNPs were synthesized by using some very common reducing reagents like ascorbic acids, potassium citrate, borohydride, lemon grass and branched polyethylenimine, etc.9-15 Different scientific groups used sodium citrate as a common reducing agent to get spherical AuNPs which was familiarly done by Turkevich et al in 1951 and thereafter systematically it was going on.16-19It was also established that throughout the reduction reaction the reductant tri-sodium citrate has various roles.20 Besides reduction of HAuCl4, tri-sodium citrate plays a fundamental role in determining the pH of the reductive reaction which gives an impact on the size of AuNPs.21 Focus should be given on in situ and seed growth processes in order to prepare spherical gold nanoparticles. It follows in situ process when nucleation and consecutive growth are accomplished in the same rection.22 On the other hand in seed growth mainly smaller paricles allows to be grown in to larger particles.18, 23 Here in this article spherical AuNPs were synthesized are nearly of a particular diameter about 20 nm. 20nm AuNPs has size (diameter) advantages in catalytic reaction shown by Aromal and coworkers. They observed that in the presence of AuNPs having diameter of 20 nm the reduction of 4-nitrophenol completed in 30 minutes.24 Some researchers show that 20nm AuNPs were up taken preferentially over the other diameter by the pancreas cancer cells which is very useful in cancer treatment and X-ray drug deliverysystem.25 Amyloidal fibril formation of beta-lactoglobulin could be inhibited by 20 nm AuNPs reported by U. C. Halder et al.26 Y.R. Lee et al investigated that 20 nm AuNPs were act as an in vitro antioxidant. They also found that antibacterial properties of the synthesized nanoparticles (AuNPs) which was very significant.27Hence 20nmAuNPs have attracted extensive attention for their multiple, unique functional properties, easy to synthesis within a small duration, vast applications and advantages of which some are above mentioned. Therefore attention is paid on synthesis and characterization of a particular diameter AuNPs i.e. 20 nm.
Materials and Methods
Reagent and chemicals
Chloroauric acid (HAuCl4) and tri-sodium citrate (all AR grade) were purchased from Sisco Research Laboratory (Mumbai, India) and Merck (Mumbai, India) respectively. These two main chemicals were used without additional purification, as received. Millipore water was used to prepare all the solutions.
Synthesis of gold nanoparticles (AuNPs)
The Chemical reduction of Chloroauric acid was done by trisodium citrate to prepare colloidal AuNPs following Frens method.28The glass apparatus which are used in the preparation were washed by aqua regia, rinsed with Millipore water and dried in oven. To synthesize AuNPs 5 ml of 38.8mM tri-sodium citrate was added quickly to a solution of 50ml of 1 mM HAuCl4 and the solution mixture was kept stirring for about 25 minutes till the colour of the solution turns wine red. The wine red solution was filtered through 25 mm syringe filter with 0.2 μm membrane and the filtrate was stored. The following two reactions are involved in the reduction process of chloroauric acid by tri-sodium citrate.

Characterization of AuNPs by UV-visible spectroscopy:
The UV absorption spectrum was taken in the 350–700 nm wave-length range at room temperature by using Shimadzu-TCC 240A UV-Vis spectrophotometer. Path length of the cubate used in this purpose was 1cm.
Measurement of AuNPs by Dynamic light scattering (DLS)
The intensity of the scattered light induces fluctuations by the nanoparticles which are diffused in the solution. As DLS is very sensitive to particle size, it is used to detect the distribution of the molecules and supramolecular aggregates.29 Different peaks were observed for different sizes of the molecules. In this experiment the measurements of DLS was done with citrate coated AuNPs employing Zetasizer Nanos(Malvern Instrument, U.K.), using 2 ml rectangular cuvette (path length 10 mm), which is equipped with and 633 nm laser. The measurement of hydrodynamic diameter of AuNPs was done at 20◦C.
X-ray diffraction (XRD)
Colloidal AuNPs containing solution was lyophilized for two days to solidify it for XRD study. Then a Brucker AXS (Model D8, WI, USA) was used to measure the XRD pattern of powered AuNPs sample. The pattern was recorded with Cu Kα radiation (λ=1.5409Å) at a scan rate 1step/0.2sec within the scanning range of two thetta (2q) from 20° to 80°. The tube voltage and the tube current were 40 Kv and 40 mA respectively in room temperature during the experiment.
Morphological characterization by TEM
A high resolution transmission electron microscope (JEOL-HRTEM-2011, Tokyo, Japan) was used for the morphological studies of AuNPs between the voltage region 80-85Kv with different magnification. The sample solution was sonicated about 60s and diluted about 50 times. A micropipette was used to drop cast of the diluted solution on a carbon coated copper grid of mesh size 300C (Pro Sci Tech). The droplet was soaked by filter paper after 20s. The grid was air dried and incubated for 6h before taking TEM images. SAED and EDX were also observed with the same sample prepared for TEM analysis.
Results and Discusion
Characterization by UV-spectroscopy
The UV-Spectra recorded from the colloidal solution (strong red colour of AuNPs) was shown by Fig. 1. This figure shows a typical Plasmon band which appeared a single but strong absorption peak centered at about 524.5 nm.
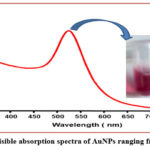 |
Figure 1: UV-visible absorption spectra of AuNPs ranging from 350-800nm. |
Dynamic Light Scattering (DLS)
DLS is normally familiar in observing the hydrodynamic size (radius) of particles within the solution which is an estimation of the aggregated particles. Relative proportion of particle size (radius) measurement was done in this section. The hydrodynamic diameter AuNPs were found ∼20nm which is represented in (Fig. 2).
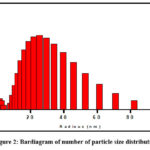 |
Figure 2: Bardiagram of number of particle size distribution. |
X-ray diffraction (XRD):
The XRD pattern was shown by Fig.3 . The four distinct diffraction peaks of 2q values at 38.2°, 44.4°, 64.1° and 77.6° represents 111, 200, 220 and 311respetively.It indicated that synthesized AuNPs are crystalline in nature. This result matched with JCPDS file no 04-0784.
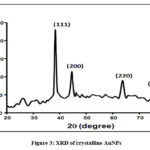 |
Figure 3: XRD of crystalline AuNPs |
Characterization of AuNPs by HRTEM and SAED
Here TEM was done to confirm the diameter of the synthesized AuNPs. It was observed that the diameter of AuNPs were of about 20 nm (Fig. 4c). The TEM analysis revealed that the synthesized AuNPs were mostly spherical in shape and well dispersed. Fig. 4 revealed the morphoplogy of spherical AuNPs with different magnification (Fig. 4a-c). Inset of Fig. 4a represents the bright SAED pattern of synthesized AuNPs which proves particles are metallic in nature.30
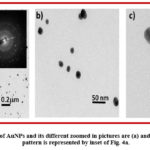 |
Figure 4: TEM pictures of AuNPs and its different zoomed in pictures are (a) and (b-c) respectively. SAED pattern is represented by inset of Fig. 4a. |
EDX analysis
The optical adsorption peak was observed at approximately 1.90, 2.30, 8.5, 9.8, 10.4, 11.5 and 13.4 keV.31-33 These are typical EDX signal for the adsorption of AuNPs due to SPR shown in (Fig. 5). Among all the above mentioned peaks 2.30 KeV displayed highest intensity which indicates the presence of spherical AuNPs and are pure in nature already mentioned by few research groups.31,32
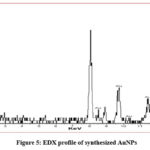 |
Figure 5: EDX profile of synthesized AuNPs. |
Discussion
There is a strong absorption paek at 524.5 nm in UV-Vis study reveals that the diameters of synthesized bare AuNPs are of ~ 20nm. This result also indicates that the particles are isotropic in shape and uniform in size. The DLS study also indicates the same result. The XRD pattern represents the production of crystalline AuNPs with definite planes. The clear TEM images are the solid proof of diameter of AuNPs which are ~20nm. SAED indicates about the crystalline nature of AuNPs and EDX gives supportive evidence about the pure spherical size AuNPs.
Conclusion
There are certain issues which need to be point out. Firstly the synthesis of metal nanoparticles of a particular diameter with accuracy is very difficult due to their stability in colloidal medium and their performance in such a complicated surroundings. Secondly to develop the possible application of AuNPs, it is very much necessary to study systematically the preparation and characterization of it (AuNPs). On the basis these two points this article should get importance because I have synthesized AuNPs (of a particular diameter) were found ~ 20nm which was proved by the above mentioned experiments. Citrate stabilized spherical AuNPs were colloidal in nature. Citrate plays dual role as a reducing reagent of HAuCl4 and stabilizer of synthesized AuNPs in that colloidal environment. Still more and more experiments needed to understand the procedure of synthesis of AuNPs of an accurate diameter and their stability in the colloidal environment.
Acknowledgement
I wish to acknowledge Hon’ble Principal, Dr. Manideep Chandra, Shibpur Dinobundhoo Institution (College) for his support and encouragement. I also like to gratefully acknowledge Prof. U. C. Halder, Department of Chemistry, Jadavpur University for his necessary guidance and cooperation regarding this work.
Conflicts of Interest
The author of this paper has no conflict of interest.
References
- Daniel, M.-C.; Astruc, D. Chem. Rev., 2004, 104, 293–346.
CrossRef - Boisselier, E.; Astruc, D. Chem. Soc. Rev., 2009, 38, 1759–1782.
CrossRef - Dreaden, E. C.; Alkilany, A. M.; Huang, X.; Murphy, C. J.; El-Sayed, M. A. Chem. Soc. Rev., 2012, 41, 2740–2779.
CrossRef - Ghosh, P.; Han, G.; De, M.; Kim, C. K.; Rotello, V. M. Adv. Drug Delivery Rev., 2008, 60, 1307–1315.
CrossRef - Kamat, P. V. J. Phys. Chem. B 2002, 106, 7729–7744.
CrossRef - Saha, K.; Agasti, S. S.; Kim, C.; Li, X.; Rotello, V. M. Chem. Rev., 2012, 112, 2739–2779.
CrossRef - Nguyen, D. T.; Kim, D. J.; Kim, K. S. Micron, 2011, 42, 207.
CrossRef - Parab, H.; Jung, C.; Woo, M. A.; Park, H. G.; J. Nanopart. Res., 2011, 13, 2173.
CrossRef - Kimling, J.; Maier, M.; Okenve, B.; Kotaidis, V.; Ballot, H.; Plech, A. J. Phys. Chem. B 2006, 110, 15700−15707.
CrossRef - Patungwasa, W.; Hodak, J. H. Mater. Chem. Phys., 2008, 108, 45−54.
CrossRef - Perez-Juste, J.; Liz-Marzan, L. M.; Carnie, S.; Chan, D. Y. C.; Mulvaney, P. Adv. Funct. Mater., 2004, 14, 571−579.
CrossRef - Lohse, S. E.; Dahl, J. A.; Hutchison, J. E. Langmuir, 2010, 26, 7504−7511.
CrossRef - Goia, D. V.; Matijevic, E. Colloids Surf., A 1999, 146, 139−152.
CrossRef - Shankar, S. S.; Rai, A.; Ankamwar, B.; Singh, A.; Ahmad, A.; Sastry, M. Nat. Mater., 2004, 3, 482−488.
CrossRef - Kim, R.; Park, H. S.; Yu, T.; Yi, J.; Kim, W.-S. Chem. Phys. Lett., 2013, 575, 71−75.
CrossRef - Turkevich, J.; Stevenson, P. C.; Hillier, J. Discuss. Faraday Soc., 1951, 11, 55−7
CrossRef - Frens, G. Nature, Phys. Sci., 1973, 241, 20−22.
CrossRef - Brown, K. R.; Natan, M. J. Langmuir, 1998, 14, 726−728.
CrossRef - Kimling, J.; Maier, M.; Okenve, B.; Kotaidis, V.; Ballot, H.; Plech, A. J. Phys. Chem. B 2006, 110, 15700−15707.
CrossRef - Ji, X. H.; Song, X. N.; Li, J.; Bai, Y. B.; Yang, W. S.; Peng, X. G. J. Am. Chem. Soc. 2007, 129, 13939−13948.
CrossRef - Patungwasa, W.; Hodak, J. H. Mater. Chem. Phys., 2008, 108, 45−54.
CrossRef - Zhao, P.; Li, N.; Astruc, D. Coord. Chem. Rev., 2013, 257, 638–665.
CrossRef - Keating, C. D.; Musick, M. D.; Lyon, A. L.; Brown, K. R.; Baker, B. E.; Pena, D. J.; Feldheim, D. L.; Mallouk, T. E.; Natan, M. J. In Nanostructured Materials: Clusters, Compositesand Thin Films; Moskovits, M., Shalaev, V. M., Eds.; ACS Symposium Series; American Chemical Society: Washington, DC, 1997; p 679.
- Aromal, S. A., Babu, K. V. D.; Philip, D. Spectrochim. Acta. A. Mol. Biomol. Spectrosc., 2012, 96, 1025–1030.
CrossRef - Trono, J. D.; Mizuno, K.; Yusa, N.; Matsukaw, T.; Yokoyama, K.; Uesaka, M. J. Radiat. Res., 2011, 52, 103–109.
CrossRef - Sardar , S.; Pal , S.; Maity , S.; Chakraborty , J.; Halder, U. C. Int. J. Biol. Macromol., 2014, 69, 137–45.
CrossRef - Basavegowda, N.; Idhayadhulla, A.; Lee, Y. R. Ind. Crop. Prod., 2014, 52, 745–751.
CrossRef - G. Frens, Nat. Phys. Sci., 1973, 241, 20.
CrossRef - Yu, W. W.; Chang, E.; Falkner, J. C.; Zhang, J.; Sl-Somali, A. M.; Sayes, C. M.; Johns, J.; Drezek, R.; Colvin, V. L. J. Am. Chem. Soc., 2007, 129, 2871–2879.
CrossRef - Deka, P.; Deka, R. C.; Bharali, P.; New J. Chem., 2014, 38,1789.
CrossRef - Elavazhagan, T.; Arunachalam K. D. Int. J. Nanomed., 2011, 6, 1265–1278.
CrossRef - Ahna, S.; Singha, P.; Janga, M.; Kima, Y-J; Castro-Aceitunoa, V.; Simub, S. Y.; Kima, Y. J.; Yang, D-C; Colloids and surfaces B: Biointerfaces, 2018, 162, 398–404.
CrossRef - Sakellari, G. I.; Hondow, N.; Gardiner, P. H. E. Chemosensors, 2020, 8, 80.
CrossRef

This work is licensed under a Creative Commons Attribution 4.0 International License.









