Isolation, Characterization of Active Constituent and Evaluation of Hepatoprotective Activity of Inula Racemosa Hook. F. Roots
1Central Facility of Instrumentation, School of Pharmaceutical Sciences, IFTM University, Moradabad, 244102, India.
2Central Facility of Instrumentation, Pharmacy Academy, IFTM University, Moradabad, 244102, India.
Corresponding Author E-mail: harpreetproctor@rediffmail.com
DOI : http://dx.doi.org/10.13005/ojc/370526
Article Received on : 22-Jul-2021
Article Accepted on :
Article Published : 19 Oct 2021
Reviewed by: Dr. dwi juli puspitasari

Second Review by: Dr. C.J. Patil

Final Approval by: Dr. Tanay Pramanik
Ayurvedic literature claims that Inula racemosa Hook. f. roots are beneficial for the liver. The study’s aim was to test the hepatoprotective effect of Inula racemosa Hook. f. roots ethanolic extract (IRE) against paracetamol (PCM) induced hepatotoxicity in rats. Silymarin (100 mg/kg/day) was used for 7 days, then PCM was orally administered (3 g/kg b.wt.) on the eighth day. 24 hours after the last PCM dosing, blood was withdrawn from the retro-orbital plexus and later on the rats were sacrificed. Aspartate Aminotransferase (AST), Alanine Aminotransferase (ALT), Alkaline Phosphatase (ALP), Albumin (ALB), Total Protein (TP), liver weight and relative liver weight were determined. Histopathology of the liver was performed. In the study, IRE was found to have comparable protective effects against PCM-induced hepatotoxicity as Silymarin. Gallic acid was isolated for the first time from Inula racemosa Hook. f. roots. IRE exhibited a hepatoprotective effect because gallic acid was present in it.
KEYWORDS:Carboxymethyl Cellulose; Hepatoprotective; Inula racemosa Hook. f. roots Silymarin
Download this article as:| Copy the following to cite this article: Kumar V, Singh H, Mishra A. K. Isolation, Characterization of Active Constituent and Evaluation of Hepatoprotective Activity of Inula Racemosa Hook. F. Roots. Orient J Chem 2021;37(5). |
| Copy the following to cite this URL: Kumar V, Singh H, Mishra A. K. Isolation, Characterization of Active Constituent and Evaluation of Hepatoprotective Activity of Inula Racemosa Hook. F. Roots. Orient J Chem 2021;37(5).Available from: https://bit.ly/2XtOx3E |
Introduction
The developed countries still depend on herbal traditional medicines1. Even in developing as well as developed countries, herbal drugs play an important role in curing various human ailments2. Herbal drugs are very popular because they are cheaper, easily available, highly efficacious and less toxic in nature3. Some of the herbal drugs are even more efficacious than synthetic drugs4. The Inula (Compositae) is well-known for its anti-inflammatory, antitumor, antimicrobial, hepatoprotective, cytotoxic properties5. It is comprised of approximately 100 species that are found throughout Europe and Asia6. Inula racemosa Hook. f. (Asteraceae) is a plant that is also known as Pushkaramula, is a traditional indian plant used to cure various human ailments. Pushkarmula is an important herb, mentioned in various Ayurvedic literature7. This herb possesses various attributes like kasari-an enemy of cough, sulahara-pain killer, sughandhika-fragrant etc8. The great sage Charaka has categorized it as hikkanigrahana-stops hiccup and svasahara-alleviates the breathlessness, asthma7. It is mentioned in Ayurvedic literatures that Inula racemosa Hook. f. roots possess hepatoprotective, cardioprotective, antihyperglycemic, analgesic and antimicrobial properties5. As a result, the study’s goal was to assess the hepatoprotective activity of Inula racemosa Hook. f. roots ethanolic extract against PCM induced hepatotoxicity in laboratory animals.
Materials and Methods
Chemicals and Instrument used for the study
The chemical used were of the purity and AR grade. The following substances were used in the study: Paracetamol RS drug was procured from SD Fine Chemical Ltd, Mumbai, India); Silymarin (Sigma Chemicals, USA); ALB test kits, ALP test kits, AST test kits, ALT test kits. The kit for TP was procured from Span Diagnostics Ltd, Surat, India. UV spectrophotometer (UV-1800, Shimadzu) was employed form analysis. Biochemical Analysis was performed on Semi Bioautoanalyser (Erba Chem 5x- Biochemistry Analyzer).
Collection and Authentication
Inula racemosa Hook. f. roots and specimen were collected from Kashmir in July 2018. Dr. Ashok Kumar, Assistant Professor, Department of Botany, IFTM University, Moradabad, India, authenticated the collected roots and plant specimens. A voucher specimen (67/SOS/BOT/2018) is preserved in herbarium section of the department for any reference.
Preparation of Inula racemosa Hook. f. roots extract
The roots were air-dried and ground into a coarse powder. Powdered roots were extracted using ethanol in the Soxhlet apparatus. The extract was concentrated in a rotary evaporator to recover the solvent before making air dried sample of extracts. Weight of airdried IRE was taken to calculate extractive yield. The airdried IRE was stored at 4 °C in refrigerator till further use for pharmacological activity evaluation.
Experimental Animals
Rats of swiss albino strain (150-200 g) as laboratory animals were taken from Institutional Laboratory, IFTM University Moradabad. Prior to start of experimentation and even during the experimentation, all laboratory animals were housed separately in polypropylene cages with 50- 60% humidity levels and a temp of 23 ± 2 °C with a 12 h diurnal period. Throughout the housing period, the rats were fed a standard pellet diet and given water. Before starting the animal based experiments, the experimental protocol was renewed and approved by Institutional Animal Ethics Committee (IAEC) as per guidelines of Committee for Control and Supervision (CPCSEA).
Experimental Protocol
Swiss albino rats have been arbitrarily divided into 5 groups of 5 rats each. Vehicle (1 percent CMC) treatment for 8 days was served to Gr-I animals and represented with name control group.
Vehicle (1 percent CMC) for 7 days was given to Gr-II animals before inducing hepatotoxicity. For inducing hepatotoxicity on the day 8th, PCM (3 g/kg b.w.) was orally administered in a single dose.
The animals belonging to Gr-III, Gr-IV and Gr-V were treated with dose of 150 mg/kg, 300 mg/kg and Silymarin 100 mg/kg for 7 days before attempting to induce hepatotoxicity on the eighth day. In order to induce hepatotoxicity, PCM (3g/kg b.w.) was administered in a single dose. The retro-orbital plexus was used to withdraw blood under mild anaesthesia after 24 hours of the last PCM dosing, and later on rats were sacrificed. Serum was separated for biochemical parameters evaluation by performing centrifugation at 3000 rpm at 4 °C for 20 min. The dissected tissue upon thoroughly washing with ice-cold saline, it was blotted and allowed to dry before weighing each group.
The relative organ weight was calculated using formula-
L=Normal (-3+0.4×D+0.04×B, 0.6)
Where
B = Weight of Body
L = Weight of Liver
D = Dose
For histopathological examination, the tissue was fixed in formal in9 .
Liver Function Test
Serum AST, ALT, ALP, ALB, AST and TP were estimated using a UV spectrophotometer (Shimadzu-1800) and a Semi Bioautoanalyser obtained from Span Diagnostics Ltd., Surat.
Histopathological Examination
Liver slides were kept in 10 % formaldehyde solution. By embedding the dissected tissue in paraffin wax, it was mounted10. After cutting, the sizes of sections were 5 mm. Eosin and Haemotoxylin dyes were used to stain the sections. Light microscope was used to observe slides. Digital camera was used to capture photomicrographs of histopathological slides11.
Isolation and Charaterization of Compound A
IRE (3g) was column chromatographed on silica gel and then eluted with various n- hexane: trifluoroethanol ratios. The IRE was then fractionated into 55 fractions using n- hexane: trifluoroethanol solvent system and monitored using TLC (Table 1). Some fractions had no spots, while others had spots. Fractions with clear spots and similar Rf values were mixed. Five fractions (31–35) were eluted. They were collected by column chromatography using the solvent system n-hexane: trifluoroethanol (40:60). These fractions have shown identical Rf value over TLC plate development with n-hexane: trifluoroethanol (0.8:1.2). As a result, they were combined (F-7) and showed the same Rf value of 0.48 in this solvent system. F-7 gave a clear spot on the TLC plate, yielding 19 mg. Spot was visualized with the help of ultravoilet light at 360 nm. Compound A was recrystallized from ethanol to form brown gleaming crystals. IR, 1H NMR spectroscopy (1H NMR and 13C NMR spectroscopy) and Mass spectroscopy were employed to characterize the isolated and recrystallized compound.
Table 1: Fractions of IRE of Inula racemosa Hook. f. roots
|
System of Solvents |
Ratio of Solvents |
Fractions obtained through Column Chromatography |
Thin Layer Chromatography |
Codes for Fractions |
|
n-hexane:trifluoroethanol ratios |
100:0 |
1-5 |
Not Clear |
F–1 |
|
n-hexane:trifluoroethanol ratios |
90:10 |
6-10 |
Not Clear |
F–2 |
|
n-hexane:trifluoroethanol ratios |
80:20 |
11-15 |
Not Clear |
F–3 |
|
n-hexane:trifluoroethanol ratios |
70:30 |
16-20 |
Tailing |
F–4 |
|
n-hexane:trifluoroethanol ratios |
60:40 |
21-25 |
No Spot |
F–5 |
|
n-hexane:trifluoroethanol ratios |
50:50 |
26-30 |
Clear |
F–6 |
|
n-hexane:trifluoroethanol ratios |
40:60 |
31-35 |
Clear Spot |
F–7 |
|
n-hexane:trifluoroethanol ratios |
30:70 |
36-40 |
No Spot |
F–8 |
|
n-hexane:trifluoroethanol ratios |
20:80 |
41-45 |
No Spot |
F–9 |
|
n-hexane:trifluoroethanol ratios |
10:90 |
46-50 |
Not Clear |
F–10 |
|
n-hexane:trifluoroethanol ratios |
0:100 |
51-55 |
Not Clear |
F–11 |
Statistical Evaluation
Findings of present study are expressed in mean ± SEM. For the determination of the level of significance, graph prism pad software was used.
Results
Hepatoprotective Activity
Extraction Yield (%) of IRE was 5.05±0.34%. IRE was black in color and with sticky consistency. Effects of standard drug (Silymarin) and different doses of IRE on biochemical parameters of liver are shown in Table 1. Relative Liver Weight of PCM treated group was significantly (p<0.01) increased to 5.61±0.42/100 g body weight (b. wt.) in comparison to Gr-I (i.e., Control Group). Relative Liver Weight of Gr-I (i.e., Control Group) was 3.11±0.05/100 g b. wt. Ethanolic extract of Inula racemosa Hook. f. roots (IRE) significantly (IRE 150 mg/kg, p<0.05 and IRE 300 mg/kg p<0.01) decreased the Relative Liver Weight to 3.58±0.20/100 g b. wt. and 3.14±0.01/100 g b. wt. respectively in comparison to Gr-II (i.e., only PCM treated group). Relative Liver Weight of Gr-II (i.e., only PCM treated group) was 5.61±0.42/100 g b. wt. Results of Gr-IV (i.e., treated with IRE 300 mg/kg) were more encouraging as the rats treated with IRE 300 mg/kg were cured significantly as shown in the Table 2. Serum AST, ALT and ALP was significantly (p<0.01) increased to 316.89±1.00 U/L, 181.51±7.0 U/L and 200.24±1.81 U/L respectively in Gr-II in comparison to Gr-I. Serum AST, ALT and ALP of Gr-I rats were 94.10±0.60 U/L, 43.09±1.83 U/L, 66.79±1.14 U/L respectively. Gr-III (treated with IRE 150 mg/kg) and Gr-IV (treated with IRE 300 mg/kg) significantly decreased AST (209.59±1.130 U/L, p<0.05 and 100.10±1.90 U/L, p<0.01), ALT (90.58±2.110 U/L, p<0.05 and 50.10±3.27 U/L, p<0.01), ALP (104.15±2.58 U/L, p<0.05 and 68.18±1.04 U/L, p<0.01) respectively in comparison to Gr-II. Gr-V (treated with standard drug (Silymarin 100 mg/kg)) significantly (p<0.01) reduced the increased levels of AST, ALT and ALP to 96.10±2.61 U/L, 48.80±1.84 U/L and 67.13±1.05 U/L respectively in comparison to Gr-II. Serum ALB and TP was significantly (p<0.01) reduced to 2.20±0.05 g/dl and 3.71±0.15 g/dl respectively in Gr-II in comparison to Gr-I (i.e., Control Group). Serum ALB and TP of Gr-I rats were 4.06±0.03 g/dl and 6.19±0.24 g/dl respectively. Gr-III (treated with IRE 150 mg/kg) and Gr-IV (treated with IRE 300 mg/kg) significantly increased serum ALB (3.54±0.18 g/dl, p<0.05 and 4.10±0.01 g/dl, p<0.01) and TP (4.80±0.17 g/dl, p<0.05 and 6.05±0.10 g/dl, p<0.01) in comparison to Gr-II. Gr- V (treated with standard drug (Silymarin 100 mg/kg)) significantly (p<0.01) increased the declined level of ALB and TP to 4.10±0.02 g/dl and 6.16±0.18 g/dl in comparison to Gr-II. Figure 1 depicts the protective effects of IRE on the histopathology of rat liver.
Table 2: Effect of ethanolic extract of Inula racemosa Hook. f. roots (IRE) and standard drug (Silymarin) on Paracetamol (PCM) induced experimental groups
|
Biochemical Parameters and Treatment Groups (n=5) |
AST(U/L) |
ALT(U/L) |
ALP(U/L) |
ALB(g/dl) |
TP(g/dl) |
Liver Weight(g) |
Relative Liver Weight
(Liver Weight/100g b.wt.) |
|
I (Control-1% CMC 1ml/kg body weight) |
94.10±0.60 |
43.09±1.83 |
66.79±1.14 |
4.09±0.03 |
6.19±0.24 |
6.10±0.23 |
3.11±0.05 |
|
II (Hepatotoxic Control- 1% CMC 1 ml/kg/ body weight + PCM 3g/kg body weight) |
316.89±1.00# |
181.51±7.0# |
200.24±1.81# |
2.20±0.05# |
3.71±0.15# |
8.83±0.33# |
5.61±0.42# |
|
III (IRE 150 mg/kg b.wt. + PCM 3g/kg body weight) |
209.59±1.130 * |
90.58±2.110 * |
104.15±2.58* |
3.54±0.18* |
4.80±0.17* |
7.00±0.60ns |
3.58±0.20* |
|
IV (IRE 300 mg/kg b.wt. + PCM 3g/kg body weight) |
100.10±1.90** |
50.10±3.27** |
68.18±1.04** |
4.10±0.01** |
6.05±0.10** |
6.19±0.21** |
3.14±0.01** |
|
V(Silymarin 100 mg/kg b.wt. + PCM 3g/kg body weight) |
96.10±2.61** |
48.80±1.84** |
67.13±1.05** |
4.10±0.02** |
6.16±0.18 |
6.12±0.22** |
3.10±0.03** |
Findings are expressed in mean±SEM. # indicates p<0.01 as compared with Gr-I; nsindicates *p<0.05,**p<0.01 in comparison to Gr-II
 |
Figure 1: Liver tissue histopathology (At 10X). Click here to View figure |
Charaterization of Compound A
IR (KBr) νmax: 3370, 3070, 2661, 1705, 1451, 1379, 1306, 871, 792, 763 cm−1; 1H NMR (δ ppm, 400 MHz, DMSO): δ 7.11 (1H, s, H-6), δ 7.14 (1H, s, H-2), sharp singlets of aromatic protons. 13C NMR (δ ppm, 100 MHz, DMSO): δ 115.3(C-6), δ 147.2 (C-5), δ 140.7 (C-4), δ 146.5 (C-3), δ 113.7 (C-2), δ 124.9 (C-1), δ 168.8 (Carbon of –COOH group); ES-MS m/z 170.07 [M+] and molecular weight was found to be 170 (C7H6O5 ) (Fig. 2).
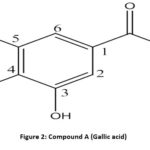 |
Figure 2: Compound A (Gallic acid) |
Discussion
Paracetamol (PCM) is a NSAID which, when taken in frequently at higher dose, cause toxicity to liver and can even cause death of experimental rats and human beings13. The serum AST, ALT and ALP level were increased; ALB and TP level were decreased. The relative liver weight increased due to hepatotoxity of liver caused by PCM. This indicates the damage of hepatocytes in liver14. Acute toxicity testing was carried out using IRE as per OECD 420. The fixed dose of 1500 mg/kg b. w. was found to be having no toxicity to rats. So, it can be considered as safe dose. Therefore, 1/10th and 1/5th of the fixed dose (1500 mg/kg b. wt.) of IRE were selected for the proposed study. Two dose levels selected were 150 mg/kg b. wt. and 300 mg/kg b. wt. IRE (300 mg/kg) significantly (p<0.01) decreased the level of serum enzymes including AST, ALT and ALP in the PCM intoxicated animals15. The findings suggested a correlation between the results of liver enzymes test and histopathological changes in photomicrographs16. Hepatic necrosis, degeneration of cells and infiltrating lymphocytes were well observed in Gr-II (Hepatotoxic Control)17. Oral administration of IRE (300 mg/kg) prevented the PCM induced changes as observed in photomicrographs of liver slides18. Results of the study suggested that IRE is potent enough to prevent the hepatic toxicity and the secondary metabolites observed accountable for the study may be phenolic acid (Gallic acid). As the IRE exhibited good hepatoprotective activity, Gallic acid may be accountable for this. Phenolic compounds are quite well known for their anti-inflammatory and antioxidant activity. They safeguard against oxidative damage by donating hydrogen or electron to free – radicals and as a result, they aid in the stabilization of cell membrane networks as well as the inhibition of the development and expression of inflammatory cytokines such as β-TGF (Transforming Growth Factor Beta), TNF-α (Tumour Necrosis Factor Alpha) and various Interleukins.
Conclusion
Based on the results of the study, researchers concluded that ethanolic extract of Inula racemosa Hook. f. roots (IRE) possess potential to protect liver against toxicity induced due to PCM in Swiss albino rats. The findings suggested that IRE reduced significant concentration of AST, ALT, ALP enzyme and on another side, it increased ALB and TP enzyme. Unwanted histopathological changes caused by PCM were also healed with the oral administration of IRE in animals. IRE might be active due to Gallic acid, which is present in the extract. The researcher succeeded in isolating and identifying Gallic acid for the first time from the roots of Inula racemosa Hook. f. The above isolates compiled with the spectral interpretations.
Acknowledgement
The authors would like to thank Management, IFTM University for providing laboratory facilities for smooth conduction of research work.
Conflicts of Interest
None
Funding Sources
None
References
- Serrano VC, McLaren B, Carrasco JC, Alday JD, Fiallos L, Amigo J, Oniandia M. Traditional ecological knowledge and medicinal plant diversity in Ecuadorian Amazon home gardens. Glob Eco Cons 2018; 17: 1-23.
CrossRef - Veeresham C. Natural products derived from plants as a source of drugs. Journal of Advan Pharm Tech Res 2012; 3(4): 200–201.
CrossRef - Staden JV, Ndhala AR, Ncube B. Ensuring quality in herbal medicines: Toxic phthalates in plastic-packaged commercial herbal products. Sou Afr J Bot 2012; 82: 60-66.
CrossRef - Sofowora A, Ogunbodede E, Onayade A. The Role of Medicinal Plants in the strategies for disease prevention. Afr J Trad Comp Alt Med 2013; 10(5): 210-229.
CrossRef - Sharma P. DravyaGuna-Vigyan (Aubhid Ausadh –Dravya), Chaukhambha Bhartiya Academy, Varanasi, India 2001; 296-298.
- Wallis T. Textbook of Pharmacognosy. 5th edtion. Published by J&A Churchill Limited London. 1997.
- Vadnere GP, Gaud RS, Singhal AK, Somani R. Effect of Inula racemosa root extract on various aspects of asthma. Pharmacol 2009; 2: 84-94.
- Firdous Q, Bhat FM, Masoodi M. Ethnopharmacology, Phytochemistry and Biological activity of Inula racemosa Hook. F: A Review. Inter J Res Ayu Pharm 2018; 9(1): 95- 102.
CrossRef - Apte KG, More A, Parab PB. Evaluation of activity of whole stem extracts of Oroxylum Indicum on paracetamol induced hepatotoxicity. Inter J Pharm Bio Sci 2013; 4(4): 255- 265.
- Cheng KC, Copper JE, Budgeon LR, Foutz CA, Van Rossum DB, Vanselow DJ, Hubley MJ, Clark DP, Mandrell DT. Comparative analysis of fixation and embedding techniques for optimized histological preparation of zebrafish. Comp Biochem Phys, Part C: Toxic pharmacol 2018; 208: 38-46.
CrossRef - Aigner T, Schmitz N, Laverty S, Kraus VB. Basic methods in histopathology of joint tissues. Osteo Cart 2010; 18: S113-S116.
CrossRef - Dakhale GN, Hiware Sachin K, Shinde Abhijit T and Mahatme Mohini S. Basic biostatistics for post-graduate students. Ind J Pharm 2012; 44(4): 435-442.
CrossRef - Bessone F. Non-steroidal anti-inflammatory drugs: What is the actual risk of liver damage? World J Gastroenterol 2010; 16(45): 5651-5661.
CrossRef - Mujahid M, Alam J, Badruddeen, Jahan Y, Bagga P, Rahman MA. Hepatoprotective potential of ethanolic extract of Aquilaria agallocha leaves against paracetamol induced hepatotoxicity in SD rats. J Trad Comp Med 2017; 7(1): 9-13.
CrossRef - Aba PE, Ozioko IE, Udem ND, Udem SC. Some biochemical and haematological changes in rats pretreated with aqueous stem bark extract of Lophira lanceolata and intoxicated with paracetamol (acetaminophen). J Comp Int Med 2014; 11(4): 273-277.
CrossRef - Hasan KMM, Tamanna N, Haque MA. Biochemical and histopathological profiling of Wistar rat treated with Brassica napus as a supplementary feed, Food Sci Hum Well 2018; 7: 77-82.
CrossRef - Ibraheem AS, El-Sayed MF, Khalil HA. Establishment of hepatitis model in rat liver induced by injecting extracted DNA: Histopathological study. The J Bas App Zoo 2016; 77: 102-111.
CrossRef - Singh H, Prakash A, Kalia AN and Majeed ABA. Synergistic hepatoprotective potential of ethanolic extract of Solanum xanthocarpum and Juniperus communis against paracetamol and azithromycin induced liver injury in rats. J Trad Comp Med 2016; 6(4): 370-376.
CrossRef

This work is licensed under a Creative Commons Attribution 4.0 International License.









