Comparative Dielectric Study of Binary Mixtures of Coriandar oil and Radish oil
Mohammad Shafi Khan1 , Vishal Singh Chandel2*
, Vishal Singh Chandel2* and Satyendra Pratap Singh3
and Satyendra Pratap Singh3
1ZHIIC, Nidholi Kalan, Etah, UP, India.
2Rajkiya Engineering College, Ambedkar Nagar, UP 224122, India.
3Department of Physics, AIAS, Amity University, Noida, UP 201313, India.
Corresponding Author E-mail: chandel.integral@gmail.com
DOI : http://dx.doi.org/10.13005/ojc/370207
Article Received on : 27-Jan-2021
Article Accepted on :
Article Published : 17 Mar 2021
The present paper deals with the dielectric study (dielectric constant, dielectric loss) of two medicinal oils, coriandar and radish oil and their binary mixtures at different temperatures and frequencies. HP 4194A impedance gain/phase analyzer and temperature controller (Julabo, model number F-25, microprocessor controlled) were used for determination of dielectric parameters and maintaining the temperature of pure oils their binary mixtures.
KEYWORDS:Binary Mixture; Coriandar Oil; Dielectric constant; Dielectric loss; Radish Oil
Download this article as:| Copy the following to cite this article: Khan M. S, Chandel V. S, Singh S. P. Comparative Dielectric Study of Binary Mixtures of Coriandar oil and Radish oil. Orient J Chem 2021;37(2). |
| Copy the following to cite this URL: Khan M. S, Chandel V. S, Singh S. P. Comparative Dielectric Study of Binary Mixtures of Coriandar oil and Radish oil. Orient J Chem 2021;37(2). Available from: https://bit.ly/3lnvJdP |
Introduction
Food is fuel for the body and keeps our mind fit and working. Adulterated food products are responsible for an abundant loss both physically and mentally. With the development in the methods of identifying the adulteration, it becomes imperative that food we consume must be pure. For example, mustard oil is used as the major cooking oil in India and it is highly recommended in food because it contains two essential fatty acids namely linolenic acid and a- linolenic acid which our body cannot make itself.
Edible oils are important in cooking of foods and in various industries such as cosmetics, pharmaceuticals, lubricants etc. Oils are used for cooking, flavor and texture to foods. The process of extraction of oils changes their characteristics upto a large extent. Various groups worked in developing empirical/semi-empirical equations to correlate their properties such as viscosity, surface tension, etc. With the help of such equations, we can predict changes in characteristic properties of oils. The chemical and physical properties of oils depend on composition, frequency, and temperature. Pace et al. reported dielectric properties of Soybean salad oil, Lard, Cottonseed cooking oil, Corn oil, Tex, Kremax, Tallow, Kremit and traditionally extracted bacon fat, Microwave rendered bacon fat (eleven fats and cooking oils) at 300 MHz, Shilpi Agarwal et al. reported dielectric behaviour of edible unsaturated oils and their binary mixtures and Lizhi et al reported dielectric properties of many edibles oils and fatty acids, our group has also been reported optical study of six oils [1-4]. Various researchers reported compositional, chemical, physical and other properties of oils under investigation [5-12].
Renata Nurzyńska-Wierdak reported effect of various growth stages of plant in evaluation of different constituents of the coriander seeds, Jazia Sriti et al reported the effect of nozzle diameter on various fatty acids and sterol on coriander cakes. Ghazanfari S et al. conducted study to investigate the effects of coriander oil on blood characteristics and other physiological measurements in broiler performance, Evelien Uitterhaegen et al done characterization of coriander oil as a source of petroselinic acid, Mostafa kamal Shams et al. reported the impact of environment on the oil content of coriander seeds, Dwisari Dillasamola et a.l reported the effects of coriander ethanol extract against phagocytosis activity and other biological activities such as percentage of leukocytes, Marta Simone M Freitas et al. reported the effect of doses of potassium from different sources on the production of fruits, its essential oil, macronutrient and linalool contents and found that on increasing potassium doses irrespective the source, the potassium content is increasing but calcium content is decreasing also it is not affecting content of nitrogen and magnesium in the fruits, Marika Pellegrini et al. presented use of coriandrum sativum seeds contents as a “green washing solutions” in food industry against control of several pathogens, Catherine Kern et al. reported soothing effect of coriander oil on skins, Hend E. Wahba et al. reported coriander oil content from seeds and coriander plant using gas chromatography–mass spectrometry (GC–MS) and reported that linalool is the main constituent of oil in case of plant waste trans-anethole is the major oil constituent than linalool [13-22].
K. L. Ahuja et al. reported fatty acid composition and oil content of various seeds (seven of radish, four of turnip and thirteen genotypes of clauiflower) and reported that both, turnip, and radish have higher oil content than cauliflower. Majority of the fatty acids are oleic, linoleic + eicosenoic and erucic acids. Nicholas Chammoun submitted a Master of Science thesis in 2009 on in the University of Georgia, Georgia and reported properties, performance and economics associated with production and commercialization of radish oilseed in context to biodiesel production. Tung-Ting Sham et al. published review on the phytochemistry and pharmacological Activities of raphani semen and reported that the seed is used in traditional chinese medicine (TCM) to treat various ailments such as constipation, chronic tracheitis, and hypertension.Galina Selyutina and Oksana Gapontseva reported component composition of radish root essential oil of Radish roots of varieties such as black radish, white radish, marushka, lebidka, troyandova, sertse drakona, margelan radish and daikon and concluded that they contain in majority hydrocarbons, sulfur containing substances, organic acids, aldehydes, esters, ketones, terpenes, alcohols and arenas and around 40 chemical compounds have been found, majority of them are variety specific only 14 substances are common to all the varieties of radish, they are nonanal, pentadekanal, dioktylftalat, dyizobutylftalat squalene, linoleic acid, linolenic acid, oleic acid, stearic acid, trycosane, tetracosane, pentacosane, hexacosane and heptacosane. Gongling Zhao et al. effect of various extraction methods such as sub-critical propane extraction (SPE), supercritical carbon dioxide extraction (SCE) and traditional solvent extraction (SE) on the yield and other physio chemical properties of radish oils. The yield from SPE method is highest and with SCE method is the lowest. Bingjun Qian et al. reported various biological and pytochemical composition, antioxidant capacity of nine China-grown radish seeds. Da Silva et al. studied ultrasound-assisted extraction of radish seed oil with methyl acetate for biodiesel production in context to yield, time of process, temperature required etc. They also concluded that erucic and oleic acids are the main fatty acids contents in the radish seed oil. Douglas Faria et al. reported radish oil as a potential candidate for bio diesel along with its extraction method. Abdelrahman B. Fadhil et al. have also reported various uses of radish seed oil specially in context to biodiesel applications. Natália Stevanato and Camila da Silva published ultrasound-assisted extraction of radish oil using ethanol as solvent. Dongyoung Lee et al. in year 2020 reported drying patterns of radish slabs under different drying methods such as, hot-air drying (HAD), microwave drying (MD), and hot-air and microwave combination drying (HMCD), Rostislav Y. Blume et al. explored the potential use of various brassicaceae family seeds for their oil as biofuel (camelina, turnip rape, oil radish and tyfon) [23-34].
Material and Methods
For dielectric study of coriander oil and raddish oil and their binary mixtures, they have been procured from local market and used without further purification.
Coriander Oil
This oil is extracted from the ripe seeds of coriander and yields approximately 0.8 – 1.0 % oil. Coriander oil is nearly colorless to pale yellow and has a sweet smell. Its viscosity is close to water. The main constituents of the oil are borneol, cineole, terpineol, linalool, cymene, dipentene, pinene, phellandrene and terpinolene. Studies showed that this oil helps in mental fatigue, migraine, muscle spasms, colds, stimulating the glandular system and nervous weakness etc.
The composition of coriander oil has been reported by Axel Diederichen et al., as Gamma-terpinene 9.0%, Geranylacetate 0.1-4.7%, Linalool 60-80%, a-terpineol 0.5%, Alpha-pinene 10.5%, g -terpinene 1-8%, Camphor 0.9-4.9%, Geraniol1.2-4.6%, Terpinen-4-ol3.0%, Limonene0.5-4%, a-pinene0.2-8.5%,Camphene1.4%,Myrcene0.2-2%,Ketones 7-9%,Linalyl acetate0-2.7% and fatty acids in coriander oil as Linoleic acid C18:216.6%, Petroselinic acid C18:168.8%, Palmitic C16:0 3.8%,Oleic acid C18:17.5% [35].
Radish Oil
It is a volatile, mustard like smell oil, extracted from radish seeds. This oil has anti bacterial properties due to presence of vinyl – ethyl – aizausant – glucksid alsingerin and rvannin compounds. It contains various enzymes, minerals, and vitamins such as A and C. Medicinal properties of this oil has benefits as a topical skin remedy, for stomach illnesses, greasy skin, and the promotion of circulation. Being diuretic innature it is useful for kidney and gall bladder problems.
Experimental Details
Sample holder
For dielectric measurement a sample holder (coaxial cylindrical) was designed and fabricated in the laboratory. The dimensions of the sample holder are given in fig.1. It is made of brass with all its conducting parts silver plated to reduce various losses and its geometrical capacitance is 2.085 pF.
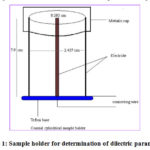 |
Figure 1: Sample holder for determination of dilectric parameters. |
Dielectric constant of the samples was calculated using relation given below-

where, a and b are the outer and inner diameter of the cylinder and h is the height of the sample in the sample holder,
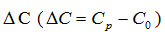
is the change in capacitance of the said sample holder, with and without oils in it.
Dielectric loss of the samples was measured by measuring first loss tangent or dissipation factor D using relation given below then dielectric loss was calculated-

where D0, C0, Dc and Cp dissipation factor and capacitance of sample holder with and without oils directly read from impedance analyser.
Dielectric loss was evaluated using following equation-

Dielectric constant and dielectric loss of coriander oil (Oil A), radish oil (Oil B) and their binary mixtures have been measured with the help of computer interfaced HP 4194A impedance gain/phase analyzer coupled with julabo F 25 temperature controller. The temperature range for study is kept between 300C to 500C, and the frequency between 10 kHz from 10 MHz. The dielectric loss and dielectric constant were determined using the relations already published by our group [36-37]. The temperature controller used in the study is Julabo F-25. All the samples investigated that is pure and binary mixtures have been designated as 1, 2, 3, 4 and 5, where sample 1 is pure oil (A), sample 2 is (75% oil A+ 25% oil B), sample 3 is (50% oil A+ 50% oil B), sample 4 is (25% oil A+ 75% oil B), and sample 5 is 100% pure oil (B).
Results and Discussion
Figurea 2a and 2b are representing the change in dielectric constant and dielectric loss of pure oils and their mixtures with log10 frequency (Hz) at indicated percentage of impurity (the second oil is treated as impurity in first oil) and at constant temperature 300C for the pure and its binary mixtures (coriander oil and radish oil). Figure 3a and 3b are showing the variation of dielectric constant and dielectric loss with percentage impurity at indicated frequencies and at constant temperature 300C while figure 4 (a, b) shows the variation of dielectric parameters with temperature at indicated percentage of impurity and at constant frequency 50kHz for the pure and its binary mixturesofcoriander oil and radish oil.
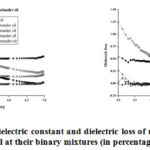 |
Figure 2: Variation of dielectric constant and dielectric loss of radish oil and coriander |
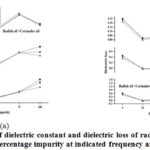 |
Figure 3: Variation of dielectric constant and dielectric loss of radish oil and coriander oil with percentage impurity at indicated frequency and 300C. |
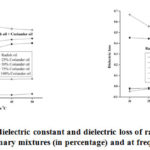 |
Figure 4: Variation of dielectric constant and dielectric loss of radish oil and coriander |
Dielectric Properties of Pure Oils and their Binary Mixtures
Frequency Dependence
Figures 1a and 1b are showing the change in dielectric constant and dielectric loss with log10 frequency at temperature 300 C for coriander oil and radish oil. It is clear from the figure 1a that the dielectric constant value of pure radish oil is less than the coriander oil. For all the samples the nature of the dielectric constant curve is almost same i.e. it decreases with increase in frequency. For the samples 2,3 & 4 the value of dielectric constant always lies between dielectric constant of sample 1 and sample 5, but for the sample 2 (coriendar oil 75% + radish oil 25%) quite large. This type of behaviour has already been published by Sorichetti, P. A. et al., [38].
Figure 1b is showing the variations of dielectric loss with log10 frequency at temperature 300C for the oils and their binary mixtures. The dielectric loss value for the samples 2, 3 and 4 are in between the dielectric loss values of sample 1 and sample 5. The dielectric loss value for sample 1 decreases very sharply up to 50 kHz frequency then it decreases slows down. The nature of graph is almost similar to all the binary mixtures of oils used in the present study. Similar type of behavior has also been reported by Lizhi et al., [39].
Composition Dependence
Figure 2a is presenting the variation of dielectric constant with percentage impurity at temperature 300C for frequency (5kHz, 10kHz, 30kHz, 50kHz, 130kHz, 330kHz, 2MHz, 4MHz, and 10MHz) for the selected oils and their binary mixtures. The dielectric constant decreases sharply initially with small impurity addition. When percentage of second oil increases up to 50% then the rate of decrease for dielectric constant is very slow with impurity addition. Therefore, we can say that the dielectric constant values decrease with increase in impurity with very slow rate. The similar nature of the curve for binary mixture of edible unsaturated oils at 300 kHz has been reported by Agrawal et al., [2]. Figure 2-b is presenting the variation of dielectric loss with percentage impurity at a constant temperature 300C for frequency (5kHz, 10kHz, 30kHz, 50kHz, 130kHz, 330kHz, 2MHz, 4MHz, and 10MHz) for the selected oils and their binary mixtures. The dielectric loss values are also decreasing with increase in percentage impurity. The value of dielectric loss up to 10% decreases sharply at all the shown frequencies and then its decrease slows down with increase in percentage impurity.
Temperature Dependence
The variation of dielectric constant with temperature at a frequency of 50 kHz are presented in figure 3a for these oils and their binary mixtures. It is observed that the dielectric constant decreases with increase in temperature for all the samples. From the figure (3-a) it is also observed that the dielectric constant for sample 1 is quite high as compared to the sample 5 and for rest of the samples, the dielectric constant values are in between sample 1 and sample 5. This type of behaviour has also been reported by Tasic, R.D., et al., [40]. The variation of dielectric loss with temperature at a frequency of 50 kHz is presented in figure 3b for the selected oils and their binary mixtures it is observed that the dielectric loss values for all the samples decreases with increase in temperature. Again, it can be seen that in figure 3b the dielectric loss values for samples 1 are moderately high as compared to the sample 5. It may be because oils consist of mixtures of esters of the trihydric alcohol.
Conclusions
The present study showed the dilectric parameters of all samples (pure and binary mixtures) decrease with increase in applied frequency. On increasing the temperature, the dielectric constant is found to decrease while revese effect is found in case of the dielectric loss. the dielectric constant and dielectric loss of binary mixtures of oils are showing unsystematic behavior. The type of study may be useful in exploring the purity of any liquid samples for example, argemone oil in mustard oil.
Conflict of Interest
There is no conflict of interest.
Acknowledgement
One of the author is thankful to TEQIP-III scheme of Rajkiya Engineering College Ambedkar Nagar, UP for providing financial assistance to the project.
References
- Pace, P. E ; Westphal, W. B ; S. A. Goldblith ; J. Food Sci., 1968, 33, 30-36.
CrossRef - Agrawal, S; and Bhatnagar, D;Indian Journal of Pure Applied Physics.2005. 43, 624-629.
- Lizhi H., Toyoda K., Ihara I.; Journal of Food Engineering. 2008, 88, 151-158.
CrossRef - Singh, S. P; Chandel, V. S; Manohar. R; European Journal of Advances in Engineering and Technology. 2017, 4(10) 744-749.
- Ramasamy, Ravi; Prakash, Maya; Bhat, Keshava K; Eur Food Res Technol.2007, 225, 367–374.
CrossRef - Yalcın, Coskuner; Karababa, Ersan; Journal of Food Engineering 2007, 80, 408–416.
CrossRef - Bhuiyan, Islam. Nazrul. Md; Begum. Jaripa; and Sultana, Mahbuba; Bangladesh J Pharmacol. 2009, 4, 150-153.
CrossRef - Shahwar, Muhammad. Khuram; El-Ghorab, Hassan. Ahmed, Anjum, Muhammad. Faqir; International Journal of Food Properties. 2012, 15, 736–747.
CrossRef - Mandal Shyamapada; Mandal, Manisha; Asian Pac J Trop Biomed. 2015, 5(6), 421–428.
CrossRef - Hani, M. Mehanna; Hussein, H.A. Said-Al Ahl; Mursy, H. Mohamed; Ngezimana, Wonder; Mudau, N. Fhatuwani; Journal of Essential Oil-bearing Plants JEOP. 2015, 18(1), 82–92.
CrossRef - Selyutina, Galina; Gapontseva, Oksana; Ukrainian Food Journal. 2016, 5(4), 653-666
CrossRef. - Priyadarshi, Siddharth; Khanum, Hafeeza; Ravi, Ramasamy; Borse, Baskarrao. Babasaheb; Naidu, Madhava. Madeneni; J Food Sci Technol. 2016, 53(3),1670–1678.
CrossRef - Nurzyńska-Wierdak, R; Acta Agrobot. 2013, 66(1), 53–60.
CrossRef - Sriti, J; Neffati, M; Msaada, K; Talou,T; and Marzouk, B; J. Chem. 2013, 1-6.
CrossRef - Ghazanfari,S; Mohammadi, Z; and Adib Moradi, M; Rev. Bras. Cienc. Avic. 2015, 17(4), 419–426.
CrossRef - Uitterhaegen, E; Klicia, A. Delbeke; Greyt, Cerny, I. P; Wim De Muriel; Evon, Philippe; Othmane Merah, Thierry Talou and Christian V. Stevens, Molecules. 2016, 21(9), 1–13.
CrossRef - Shams, M; Ramezani, M; Esfahan,S. Z; Esfahan, E. Z; Dursun, A; and Yildirim, E; Indian J. Sci. Technol., 2016, 9(6), 7–10.
CrossRef - Dillasamola, D; Aldi,Y; and Kolobinti, M; Pharmacogn. J. 2019, 11(6), 1290–1298.
CrossRef - Freitas, Marta Simone M; Gonçalves, Ygor de S; Lima, Thaísa C; Santos, Paulo Cesar dos; Peçanha, Diego A; Vieira, Marlene E; Carvalho, Almy JC; Vieira, de Ivo José C; Hortic. Bras. 2020, 38(3), 268–273.
CrossRef - Pellegrini, Marika; Rossi, Chiara; Palmieri, Sara; Maggio, Francesca; Chaves-López, Clemencia; Sterzo, Claudio Lo; Paparella, A; De Medici, Dario; Ricci, Antonella; and Serio, Annalisa; Front. Sustain. Food Syst. 2020, 4, 1–9.
CrossRef - Kern, C; Gombert, C; Roso, A; and Garcia, C; OCL – Oilseeds fats, Crop. Lipids. 2020, 27, 1-7.
CrossRef - Wahba, H. E; Abd Rabbu, H. S; and Ibrahim, M. E. Bull. Natl. Res. Cent. 2020, 44(1), 1-7.
CrossRef - Ahuja, K.L; Singh, Hari; Raheja, R.K; and Labana, K.S; Foods for Human Nutrition. 1987, (37), 33-40.
CrossRef - Chammoun, Nicholas; A Thesis: Properties, Performance, And Economics of Raphanus Sativus (Oilseed Radish) Biodiesel, Submitted to the Graduate Faculty of the University of Georgia, 2009.
- Sham, Tung-Ting; Yuen, Ailsa Chui-Ying; Ng, Yam-Fung; Chan, Chi-On; Kam-WahMok, Daniel; and Chan, Shun-Wan; Evidence-Based Comple. and Alternative Medicine, 2013, Article ID 636194. 1-16.
CrossRef - Selyutina, Galina; Gapontseva, Oksana; Ukrainian Food Journal. 2016, 5(4), 653-666.
CrossRef - Zhao, Gongling; Ren, Yupeng; and Ma, Hanjun; Tropical Journal of Pharmaceutical Research. 2017, 16(1), 165-169.
CrossRef - Qian, Bingjun; Pan, Yueping; Cai, Zhan; Jing, Pu; Journal of Applied Botany and Food Quality. 2017, 90, 315 – 322.
- Silva, Da; Garcia, Santos dos; Arroyo, P. A; & da Silva, C; The Canadian Journal of Chemical Engineering. 2017, 95 (11), 2142–2147.
CrossRef - Faria, Douglas; Santos, Fernando; Machado, Grazielle; Lourega, Rogério; Eichler, Paulo; Souza de, Guilherme; and Lima, Jeane; AIMS Energy. 2018, 6(4), 551–565.
CrossRef - Fadhil, Abdelrahman B; Nayyef, Akram W; & Al-Layla, Neam M.T; Energy Sources, Part A: Recovery, Utilization, and Environmental Effects. 2019, 1-12.
- Stevanatoa, Natália; da Silvaa, Camila; Industrial Crops & Products. 2019, 132, 283–291.
CrossRef - Lee, Dongyoung; Lohumi, Santosh; Cho, Byoung-Kwan; Lee, Seung Hyun; and Jung, Hyunmo; Foods. 2020, 9(8), 1-18.
CrossRef - Blume, Rostislav Y; Lantukh, Genadiy V; Levchuk, Iryna V; Lukashevych, Kostyantyn M; Rakhmetov, Dzhamal B; and Blume, Yaroslav B; The Open Agriculture Journal. 2020, 14, 299-320.
CrossRef - Axel, D; Coriander.IstEdition, International Plant Genetic Resources Institute. (IPGRI), 1996, 1–88.
- Khan, Mohammad. Shafi; Chandel, V. S; JPAS. 2010, 16, 69-75.
- Singh, S. P; Chandel, V. S; Manohar, R; Journal of Ayurveda and Integrative Medicine. 2017. 9(1), 53–57.
CrossRef - Sorichetti, P.A; Romano, D. S; Physics and Chemistry of Liquids. 2005, 43(1), 37–48.
CrossRef - Lizhi, H; Toyoda, K; Ihara, I; Journal of Food Engineering. 2010, 96, 167–171.
CrossRef - Tasic, R. D; Klofutar ; Acta Chim. Slov. 1999, 46(4), 511-521.

This work is licensed under a Creative Commons Attribution 4.0 International License.









