Reactions of MoCl5 and MoO2Cl2 with 4-Phenylimidazole-2-Thiol and 2-Thiazoline-2-Thiol
1Research Scholar registered with Punjab Technical University, Kapurthala-INDIA.
2Department of Applied Chemistry,GianiZail Singh Campus College of Engineering and Technology, MRSPTU,Dabwali Road, Bathinda-151001-INDIA
.
3Department of Chemistry Maharaja Ranjit Singh Punjab Technical University'
Dabwali Road, Bathinda-151001-INDIA.
Corresponding Author E-mail: gursharans82@gmail.com
DOI : http://dx.doi.org/10.13005/ojc/360611
Article Received on : 15-10-2020
Article Accepted on :
Article Published : 30 Dec 2020
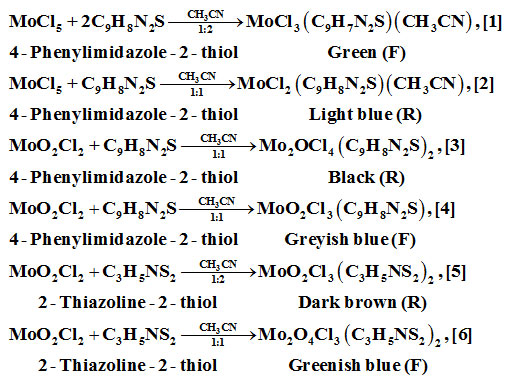
Reactions of MoCl5/MoO2Cl2 with 4-phenylimidazole-2-thiol/2-thiazoline-2-thiol in CH3CNsolvent in 1:1/1:2 molar ratios have been carried out at room temperature. Products obtained MoCl3(C9H7N2S)(CH3CN), [1];MoCl2(C9H7N2S)(CH3CN), [2]; Mo2OCl4(C9H8N2S)2, [3] and Mo4O2Cl12(C9H7N2S)4, [4]; MoO2Cl3(C3H5NS2)2, [5] and Mo2O4Cl3(C3H5NS2)2, [6] have been analyzed and characterizedby elemental analysis, FTIR, 1H NMR and LC-MS techniques. Compounds being moisture and air sensitive, these have been prepared in inert atmosphere using vacuum line and liquid nitrogen cooled traps. Fragments obtained in LC-MS spectra support the formulae derived.
KEYWORDS:FTIR; 1H NMR and LC-MS fragments; MoCl5, MoO2Cl2; 4-Phenylimidazole-2-Thiol, 2-Thiazoline-2-Thiol
Download this article as:| Copy the following to cite this article: Rani D, Singh G, Sharma S. Reactions of MoCl5 and MoO2Cl2 with 4-Phenylimidazole-2-Thiol and 2-Thiazoline-2-Thiol. Orient J Chem 2020;36(6). |
| Copy the following to cite this URL: Rani D, Singh G, Sharma S. Reactions of MoCl5 and MoO2Cl2 with 4-Phenylimidazole-2-Thiol and 2-Thiazoline-2-Thiol. Orient J Chem 2020;36(6). Available from: https://bit.ly/2J31D0D |
Introduction
Reactions of MoCl5 and MoO2Cl2 with various ligands have been reported in the literature. Earlier, MoCl5 reactions with 1, 4-diaminobutane, potassium phthalimide, pyrazole, 2-mercaptopyridine-N-oxide sodium, imidazole, 2-methylpyridine, 3-methylpyridine, 4-methtylpyridine, succinimide, 2-thiazoline-2-thiol have been reported1, 3-4, 6-7 by the author. Reactions of MoO2Cl2with 1, 3-diaminopropane, 1, 4-diaminobutane, 1, 3-propandiol, imidazole, pyrazole, acetamide, succinimide, potassium phthalimide, 2-thiazoline-2-thiol have also been reported1-3, 6-7 by the author.
In the current paper, reactions of 4-phenylimidazole-2-thiol/2-thiazoline-2-thiol with MoCl5/MoO2Cl2 in CH3CN solvent at room temperature have been carried out to study addition, substitution, reduction, rearrangement or polymerization processes occurring.
1H NMR, FTIR of the compounds synthesized have been studied to determine the bonding of the ligands to Mo. Fragmentation pattern of the compounds observed in LC-MS mass spectra support molecular formulae derived.
Aim of Investigation
Heterocyclic thioamides have N and S-donor ligands which are known to form different types of coordination compounds. They are used in analysis and corrosion control. They are biologically active. N-methylimidazoline-2-thione and other thioamides are used as anti-thyroidal agents8. Heterocyclic compounds having imidazothiazole structural unit are biologically active9. They have the ability to inhibit or activate many enzymes and receptors10-13. They act as antitumor14-19, antimicrobial20-21, antidiabetic22, diuretic23, antihelmintic24 and fungicidal25 compounds.
Molybdenum compounds containing 2-thiazoline-2-thiol have been prepared by the author2, 5-6. Metal complexes with sulphur containing ligands have many biochemical applications26-28.
Materials and Methods
MoO2Cl2, MoCl5 and 2-thiazoline-2-thiol were purchased from Sigma-Aldrich, USA and used as such.
Mo and Cl have been estimated gravimetrically by oxinate method29 and silver chloride method29,respectively. Other elements were analysed using Thermo Finnigan Elemental Analyzer. Perkin-Elmer 400 FTIR Spectrometer,in KBr disks was used to record spectra in the range 4000 – 400 cm-1.BruckerAvance-II 400 NMR in DMSO-d6 was used for obtaining 1H-NMR spectra. WATERS, Q-TOF Micromass LC-MS (UK) was used for LC-MS spectra in the range 0 – 1100 m/z. These facilities were availed at SAIF/CIL, Panjab University, Chandigarh (India).
Synthesis of Compounds [1] – [6]
Pressure stabilized dropping funnel having teflon stop-cock was connected to a 100 ml flask. For stirring, a magnetic bead was placed in the flask. Apparatus was connected to vacuum line and dried by heating. On cooling, apparatus was flushed with oxygen purged dry nitrogen gas. Known amount of MoO2Cl2 or MoCl5 was placed in the flask along with dry CH3CN solvent. 4-phethylimidazole-2-thiol or 2-thiazoline-2-thiol was placed in equimolar or 1:2 molar amount along with CH3CN solvent in dropping funnel. Solution from the dropping funnel was added to MoO2Cl2 or MoCl5 placed in bottom flask at room temperature with continuous stirring. Compounds thus prepared were filtered under reduced pressure through filtration unit. Compounds prepared have sensitivity to air and moisture. On exposure to air and moisture, their colour changes to blue. All procedures and work up have been done in vacuum line using oxygen purged under dry nitrogen gas. Moisture and oxygen were further removed using liquid nitrogen cooled traps.
Rearrangement and disproportionation have occurred during the reactions. F/R means filtrate/residue yielding the product.
Analytical Studies
Table 1: (Elemental Analysis)
|
Compounds (Colour/Formula Mass) |
% Observed (Theoretical) |
|||||
|
Mo |
Cl |
C |
H |
N |
S |
|
|
MoCl3(C9H7N2S)(CH3CN), [1] (Green/418.5) |
23.76 (22.93) |
24.76 (25.44) |
30.22 (31.54) |
03.01 (02.38) |
09.41 (10.03) |
7.31 (7.64) |
|
MoCl2(C9H7N2S)(CH3CN), [2] (Light blue/383.0) |
25.35 (25.06) |
17.73 (18.53) |
33.66 (34.46) |
02.91 (02.61) |
10.08 (10.96) |
9.21 (8.35) |
|
Mo2OCl4(C9H8N2S)2, [3] (Black/702.0) |
28.03 (27.35) |
21.00 (20.22) |
29.99 (30.76) |
02.61 (02.27) |
07.32 (07.97) |
8.35 (9.11) |
|
Mo4O2Cl12(C9H7N2S)4, [4] (Greyish blue/1546.0) |
25.63 (24.83) |
28.46 (27.55) |
27.07 (27.94) |
02.77 (01.81) |
06.86 (07.24) |
7.50 (8.27) |
|
MoO2Cl3(C3H5NS2)2, [5] (Dark brown/472.5) |
21.16 (20.31) |
22.58 (22.53) |
14.56 (15.23) |
02.08 (02.11) |
05.62 (05.92) |
26.99 (27.08) |
|
Mo2O4Cl3(C3H5NS2)2, [6] (Greenish blue/600.5) |
32.56 (31.97) |
17.40 (17.73) |
11.32 (11.99) |
02.28 (01.67) |
04.60 (04.66) |
20.47 (21.31) |
Ftir Spectra
There is an increase of 145, 149, 150 & 148 cm-1 in υ(N-H) of 4-phenylimidazole-2-thiol30-32in [1], [2], [3] & [4], respectively (Table-2) showing the presence of N-H group. There is no absorption in the region 2550-2600 cm-1 of [1], [2], [3] & [4] corresponding to υ(S-H), conveying absence of S-H group.υ(C=S) are absent in these compounds. υ(C=S) is observed at a lower wave number than υ(C=O), because C=S bond is weaker and less polar than C=O bond.C=S absorptions are less intense than those of C=O group. Absorption at 762, 761, 762 & 762 cm-1 in [1], [2], [3] &[4], respectively is associated with υ(C-S). Presence of υ(C-S) may be due to formation of Mo-S bond.
Table 2: (Infrared Absorptions in cm−1)
|
Assignment |
C9H7N2S (4-Phenylimidazole-2-thiol)30-32 |
[1] |
[2] |
[3] |
[4] |
|
υ(N-H) |
3129, 3248 s |
3393.7 v. s. |
3396.1 v. s. |
3396.6 v. s. |
3397.7 v. s. |
|
υ(S-H) |
— |
— |
— |
— |
|
|
υ(C=C), υ(C=N) |
1558, 1500, 1465 |
1623.2 s, 1593.2 sh, 1457.2 m |
1621.3 s, 1459.1 m |
1619.8 s, 1597.3 sh, 1456.9 w |
1621.7 s, 1598.2 sh, 1456.6 w |
|
υ(C=S) |
1261, 1109 |
—- |
—- |
—- |
—- |
|
υ(C-S) |
780 |
762.4 s |
761.7 s |
762.9 s |
762.2 s |
|
υ(Mo-S)33 |
421 |
— |
— |
— |
— |
|
υterminal(Mo=O)34-36 |
— |
— |
— |
981.3 s |
981.7 s |
Thiol-thionetautomerism is typical of imidazole-2-thiones32. There is a decrease in υ(Mo=O) which shows S→Mo coordination37 of 4-phenylimidazole-2-thiol in a direction trans to Mo=O bond. This shows that 4-phenylimidazole-2-thione reacted in a thiol form.
There is an increase of 285 &zero cm-1 in υ(N-H) of 2-thiazoline-2-thiol in [5] & [6], respectively (Table-3). The spectrum does not show absorption around 2710 cm-1 conveying S-H group is absent in [5] & [6]. Presence of υ(C=S) at 1308 & 1309 cm-1 in [5] & [6], respectively and further, absence of υ(C=N) indicate that S-H bond is missing in these compounds. Ligand is attached in thio-keto form in them. Bonding of ligand seems to be through S→Mo coordinate bond.
Absorption at 963 cm-1 in [5] correspondsto terminal υ(Mo=O)34-36. There is a decrease in υ(Mo=O) which shows coordination37 of 2-thiazoline-2-thiolto Mo through S atom in a direction trans to Mo=O bond. Bands at 983& 960 cm-1 in [6] indicate the presence of cis-MoO22+ core38.
Table 3: (Infrared Absorptions in cm−1)
|
Assignment |
C3H5NS22-Thiazoline-2-thiol30-32 |
[5] |
[6] |
|
υ(N-H) asym. |
3145 |
3429.7 vs |
3145.0 s |
|
υ(S-H) |
2709 |
—- |
—- |
|
υ(C=N) |
1518 s |
—- |
—- |
|
υ(C=S) |
1300 m |
1308.4 s, |
1309.8 sh, |
|
υring(C-N) |
1260 sh |
1254.8 vw |
1256.4 w |
|
υsym.(C-N) |
1218 |
1202.5 m |
1208.6 m |
|
υ(Mo-N) |
—- |
458.6 sh |
460.1 sh |
|
υ(Mo-S) |
421 |
— |
— |
|
υterminal(Mo=O)34-36 |
—- |
963.0 s |
— |
|
cis-MoO22+ core38 υ(Mo=O) |
—- |
— |
984.0 sh, 959.0 vs |
1H NMR Spectra
4-Phenylimidazole-2-thiol39, 40 has N-H peak at 13.00 ppm. Since-OH, -NH2, -SH are labile protons and spectrum is taken in some solvent, so they have no characteristic chemical shift. No N-H peak has been observed in [1], [2], [3] & [4] (Table-4). 4-Phenylimidazole-2-thiol has S-H peak at 12.15 ppm. No S-H peak has been observed in [1], [2], [3] & [4]. This indicates the absence of S-H group in [1], [2], [3] & [4]. There has been upfield shift of H-5 in all the four compounds. All the ring protons of phenyl group have also shown up field shift in all the four compounds. Presence of CH3CN in [1] &[2] has been inferred by the absorptions at 2.05 &1.98 ppm, respectively.
Table 4: (1H NMR absorptions in ppm)
|
Assignment |
C9H7N2S(4-Phenylimidazole-2-thiol) |
[1] |
[2] |
[3] |
[4] |
|
N-H |
13.00 |
— |
— |
— |
— |
|
S-H |
12.15 |
— |
— |
— |
— |
|
H-5 |
7.51 |
7.47 |
7.41 |
7.33 |
7.39 |
|
Phenyl H-2, H-6 |
8.13 |
7.90 |
7.86 |
7.76 |
7.77 |
|
Phenyl H-3, H-5 |
7.51 |
7.47 |
7.41 |
7.33 |
7.45 |
|
Phenyl H-4 |
7.41 |
— |
— |
— |
— |
|
CH3CN |
— |
2.05 |
1.98 |
— |
— |
N-H absorptions have not been observed in [5] & [6] (Table-5). Other protons have shown downfield shift in them showing S→Mo coordination.
Table 5: (1H NMR absorptions in ppm)
|
Assignment |
C3H5NS2 2-Thiazoline-2-thiol41 |
[5] |
[6] |
|
CH2 attached to N |
3.33 (2H) |
3.27 |
3.41 |
|
CH2 attached to S |
3.56 (2H) |
3.88 |
3.83 |
|
N-H |
7.43 (1H) |
— |
— |
|
S-H |
— |
— |
LC-MS Mass Spectra42
Fragmentation pattern obtained below has been used to derive the formulae (Table-6, 7). m/z values have been given below of the fragments.
 |
Table 6: (Fragmentation) Click here to View table |
Table 7: values have been given below of the fragments.
|
Comp. |
Fragment |
Calculated35 m/z |
Recorded m/z |
Rel. abundance |
|
[1] |
[C9H8N2S]+ |
176.04 |
177.09 |
40% |
|
[MoCl2(C9H7N2S)(CH3CN)]+ |
384.91 |
383.18 |
5% |
|
|
[MoCl2(C6H5)(CH3CN)]+ |
285.90 |
286.13 |
30% |
|
|
[MoCl(C6H5)(CH3CN)]+ |
250.93 |
252.14 |
10% |
|
|
[MoCl2(C9H8N2)(CH3CN)]+ |
352.93 |
351.14 |
100% |
|
|
[2] |
[MoCl2(C9H8N2S)(CH3CN)]+ |
384.91 |
383.18 |
6% |
|
[C9H8N2S]+ |
176.04 |
177.09 |
41% |
|
|
[C9H8N2]+ |
144.06 |
145.12 |
7% |
|
|
[MoCl2(C9H8N2)(CH3CN)]+ |
352.93 |
351.14 |
100% |
|
|
[MoCl2(C6H5)(CH3CN)]+ |
285.90 |
286.13 |
7% |
|
|
[3] |
[C9H8N2S]+ |
176.04 |
177.02 |
28% |
|
[Mo2OCl4]+ |
351.68 |
350.99 |
100% |
|
|
[Mo2OCl4(C9H7N2S)] |
526.71 |
525.01 |
40% |
|
|
[Mo2OCl4(C9H7N2S)2]+ |
701.74 |
699.00 |
2% |
|
|
[4] |
[C9H8N2S]+ |
176.04 |
177.03 |
28% |
|
[Mo2OCl4]+ |
351.68 |
351.02 |
78% |
|
|
[Mo2OCl4(C9H7N2S)] |
526.71 |
525.01 |
100% |
|
|
[Mo2OCl4(C9H7N2S)2]+ |
701.74 |
699.06 |
40% |
|
|
[Mo4O2Cl8(C9H7N2S)]+ |
878.39 |
873.12 |
3% |
|
|
[Mo4O2Cl8(C9H7N2S)2]+ |
1053.42 |
1049.14 |
<1% |
|
|
[Mo4O2Cl8(C9H7N2S)3]+ |
1228.46 |
1223.15 |
<1% |
|
|
[Mo4O2Cl8(C9H7N2S)4]+ |
1403.48 |
1397.18 |
<1% |
|
|
[5] |
[MoO2Cl3(C3H4NS2)2]+ |
470.75 |
470.85 |
4% |
|
[C3H5NS2]+ |
118.98 |
119.98 |
8% |
|
|
[MoO2]+ |
129.89 |
129.03 |
28% |
|
|
[MoO2S]+ |
161.86 |
161.01 |
8% |
|
|
[C3H4NS2SNH4C3]+ |
203.98 |
204.95 |
100% |
|
|
[C3H5NS2Cl2]+ |
188.92 |
188.99 |
44% |
|
|
[6] |
[MoO2Cl3(C3H4NS2)2]+ |
470.75 |
470.85 |
2% |
|
[C3H5NS2]+ |
118.98 |
120.00 |
1% |
|
|
[MoO2]+ |
129.89 |
129.05 |
25% |
|
|
[MoO2S]+ |
161.86 |
161.01 |
20% |
|
|
[C3H5NS2Cl2]+ |
188.92 |
189.00 |
86% |
|
|
[C3H4NS2SNH4C3]+ |
203.98 |
204.97 |
100% |
|
|
[MoO2Cl(C3H4NS2)2]+ |
400.82 |
399.06 |
3% |
Conclusion
There is no absorption in the region 2550-2600 cm-1 of [1], [2], [3] & [4] corresponding to υ(S-H), showing absence of S-H group. υ(C=S) stretchings are absent in [1], [2], [3] & [4]. Absorptions at 762, 761, 762& 762 cm-1 in [1], [2], [3] & [4], respectively suggestpresence of υ(C-S) of Mo-S bonds. Presence of υ(C=S) at 1308& 1309 cm-1 in [5]& [6], respectively and further, absence of υ(C=N) in [5] & [6], respectively indicate that S-H bond is missing. Bonding of ligand seems to be through S→Mo coordinate bond.
No characteristic S-H chemical shift has been observed in all the six compounds, indicating that either C=S is present or Mo-S is present in these compounds. Presence of CH3CN in [1] & [2] has been inferred due to presence of 1H NMR peak of CH3CN in them.
LC-MS spectra support the presence of particular ligands in these compounds and their proposed formulae.
Acknowledgements
We acknowledge our thanks to SAIF/CIL, Panjab University, Chandigarh (India) for extending us the characterising facility for LC-MS, elemental analysis, 1H-NMR & FTIR. Our thanks are to Campus Director, GZS CCET, MRSPTU,Bathinda, Punjab (India) for their financial support to this project.
Conflict of Interest
It is declared that the authors have no conflict of interest.
References
- Singh, G.; Mangla, V.; Goyal, M.; Singla, K.; Rani, D., American International Journal of Research in Science, Technology, Engineering & Mathematics., 2014, 8(2), 131-136.
- Singh, G.; Mangla, V.; Goyal, M.; Singla, K.; Rani, D., American International Journal of Research in Science, Technology, Engineering & Mathematics., 2015,9(1), 25-33.
- Singh, G.; Mangla, V.; Goyal, M.; Singla, K.; Rani, D., American International Journal of Research in Science, Technology, Engineering & Mathematics., 2015,10(4), 299-308.
- Singh, G.; Mangla, V.; Goyal, M.; Singla, K.; Rani, D.; Rakesh, K., American International Journal of Research in Science, Technology, Engineering & Mathematics., 2015,11(2), 158-166.
- Singh, G.; Mangla, V.; Goyal, M.; Singla, K.; Rani, D., International Congress on Chemical, Biological and Environmental Sciences.,May 7-9, Kyoto (Japan)., 2015, 930-942.
- Singh, G.; Mangla, V.; Goyal, M.; Singla, K.; Rani, D.; Kumar, R., American International Journal of Research in Science, Technology, Engineering & Mathematics., 2016, 16(1), 56-64.
- Singh, G.; Kumar, R., American International Journal of Research in Science, Technology, Engineering & Mathematics., 2018, 22(1), 01-08.
- Owczarzak, A. M.; Kubicki, M., Acta Crystallographica, Section E., 2012, E68, o1686. doi:10.1107/S1600536812020090.
CrossRef - Al, R. K. A.; Abdel, A. H. A., Molecules., 2010, 15, 3775-3815.
CrossRef - Borhani, D. W.; Calderwood, D. J.; Frank, K. E. H.; Davis, M.; Josephsohn, N. S.; Skinner, B. S., WO Pat. 2008/063287, 2008.
- Fidanze, S. D.; Erickson, S. A.; Wang, G. T.; Mantei, R.; Clark, R. F.; Sorensen, B. K.; Bamaung, N. Y.; Kovar, P.; Johnson, E. F.; Swinger, K. K.; Stewart, K. D.; Zhang, Q.; Tucker, L. A.; Pappano, W. N.; Wilsbacher, J. L.; Wang, J., Sheppard, G. S.; Bell, R. L.; Davidsen, S. K.; Hubbard, R. D., Bioorg. Med. Chem. Lett., 2010, 20, 2452-2455.
CrossRef - Emmitte, K. A.; Wilson, B. J.; Baum, E. W.; Emerson, H. K.; Kuntz, K. W.; Nailor, K. E.; Salovich, J. M.; Smith, S. C.; Cheung, M.; Gerding, R. M.; Stevens, K. L.; Uehling, D. E.; Jr. Mook, R. A.; Moorthy, G. S.; Dickerson, S. H.; Hassell, A. M.; Leesnitzer, M. A.; Shewchuk, L. M.; Groy, A.; Rowand, J. L.; Anderson, K.; Atkins, C. L.; Yang, J.; Sabbatini, P.; Kumar, R.,Bioorg. Med. Chem. Lett., 2009, 19, 1004-1007.
CrossRef - Andreani, A.; Burnelli, S.; Granaiola, M.; Guardigli, M.; Leoni, A.; Locatelli, A.; Morigi, R.; Rambaldi, M.; Rizzoli, M.; Varoli, L.; Roda, A.,Eur. J. Med. Chem., 2008, 43, 657-661.
CrossRef - Andreani, A.; Rambaldi, M.; Andreani, F.; Bossa, R.; Galatulas, I., Eur. J. Med. Chem.,1988, 23, 385-389.
CrossRef - Andreani, A.; Rambaldi, M.; Locatelli, A.; Bossa, R.; Fraccari, A.; Galatulas, I., Pharm. Acta Helv., 1993, 68, 21-24.
CrossRef - Andreani, A.; Bonazzi, D.; Rambaldi, M., Arch. Pharm., 1982, 315, 451-456.
CrossRef - Andreani, A.; Rambaldi, M. M.; Locatelli, A.; Bossa, R.; Fraccari, A.; Galatulas, I., J. Med. Chem.,1992, 35, 4634-4637.
CrossRef - Ding, H.; Chen, Z.; Zhang, C.; Xin, T.; Wang, Y.; Song, H.; Jiang, Y.; Chen, Y.; Xu, Y.; Tan, C.,Molecules., 2012, 17, 4703-4716.
CrossRef - Abdelwareth, S. A. O.; Al, D. A.; Al, M. M. A.; Adra, C. N.; Aboul, F. T.,Eur. J. Med. Chem., 2010, 45, 2689-2694.
CrossRef - Poorrajab, F.; Ardestani, S. K.; Emami, S.; Behrouzi F. M.; Shafiee, A.; Foroumadi, A.,Eur. J. Med. Chem., 2009, 44, 1758-1762.
CrossRef - Khalaj, A.; Nakhjiri, M.; Negahbani, A. S.; Samadizadeh, M.; Firoozpour, L.; Rajabalian, S.; Samadi, N.;Faramarzi, M. A.; Adipour, N.; Shafiee, A.; Foroumadi, A., Eur. J. Med. Chem., 2011, 46, 65-70.
CrossRef - Vu, C. B.; Bemis, J. E.; Disch, J. S.; Ng, P. Y.; Nunes, J. J.; Milne, J. C.; Carney, D. P.; Lynch, A. V.; Smith, J. J.; Lavu, S.; Lambert, P. D.; Gagne, D. J.; Jirousek, M. R.; Schenk, S.; Olefsky, J. M.; Perni, R. B., J. Med. Chem., 2009, 52, 1275-1283.
CrossRef - Andreani, A.; Rambaldi, M.; Mascellani, G.; Rugarli, P., Eur. J. Med. Chem. 1987, 22, 19-22.
CrossRef - Amarouch, H.; Loiseau, P. R.; Bacha, C.; Caujolle, R.; Payard, M.; Loiseau, P. M.; Bories, C.; Gayral, P., Eur. J. Med. Chem., 1987, 22, 463-466.
CrossRef - Gupta, G. D.; Jain, K. K.; Gupta, R. P.; Pujari, H. K.;Indian J. Chem., Sect. B:., Org. Chem. Incl. Med. Chem., 1983, 22, 268.
CrossRef - Kumaresan, K. L.; Lu, S.; Wen, Y.S.; Hwu, J. R., Organometallics, 1994, 13, 3170–3176.
CrossRef - Nagai, K.; Carter, B.J.; Xu, J.; Hecht, S. M.,J. Am. Chem. Soc., 1991, 113, 5099-5100.
CrossRef - Lobana, T.S.; Bhatia, P. K., J. Sci. Ind. Res., 1989, 48, 394-401.
- Vogel, A. I.,A text book of Quantitative Inorganic Analysis; John Wiley and Sons: New York, (Standard methods), 1963.
- Jolley, J.; Cross, W. I.; Pritchard, R. G.; McAuliffe,C. A.; Nolan, K. B., Inorganica Chimica Acta, 2001, 315, 36-43.
CrossRef - Kahn, E. S.; Rheingold, A. L.; Shupack, S. I., J. Crystallographic and Spectroscopic Research, 1993, 23(9), 697-710.
CrossRef - Trzhtsinskaya, B. V.; Abramova, N. D.,Sulphur Reports, 1991, 10(4), 389-430.
CrossRef - Liang Y. Q.; Zhao W. Y.; XU W. Q., Acta Chimica Sinica, 1986, 1126, 42-47.
- Barraclough, C. G.; Kew, D. J., Australian J. Chem., 1970, 23, 2387-2396.
CrossRef - Ward, B. G.; Stafford, F. E., Inorg. Chem.,1968, 7, 2569.
CrossRef - Bodo, H. H.; Regina., Z. Chem., 1976, 16, 407.
CrossRef - Abramenko, V. L.; Sergienko, V. S., Russian J. Inorg. Chem., 2009, 54(13), 2031-2053.
CrossRef - Abramenko, V. L.; Sergienko,V. S.; Churakov, A. V., Russian J. Coord. Chem., 2000,26(12), 866-871.
CrossRef - Karpov, M. V.; Davidovich, P. B.; Orlova, D. D.; Belyaev, A. N.; Garabadzhiu, A. V., Russian Journal of General Chemistry., 2015, 85(1), 206–207.
CrossRef - http://www.molbase.com/en/hnmr_6857-34-7-moldata-838140.html#tabs.
- http://www.molbase.com/en/name-2-Thiazolidinethione.html
- http://www.sisweb.com/referenc/tools/exactmass.html.

This work is licensed under a Creative Commons Attribution 4.0 International License.










