Synthesis of 3- Ferrocenyl Isocoumarins
1Department of Chemistry, Jamshedpur Cooperative College, Jamshedpur 831001, India.
Corresponding Author E-mail: neetasinha175@gmail.com
DOI : http://dx.doi.org/10.13005/ojc/360527
Article Received on : 22-08-2020
Article Accepted on : 23-09-2020
The derivatives of 3-ferrocenyl isocoumarin were synthesized by the condensation of substituted homothphalic anhydride with ferrocene using phosphoric acid or anhydrous aluminium chloride as cyclising agent. Substituted homophthalic acid did not condense with ferrocene so homophthalic acids were converted into their anhydride and then allowed to react with ferrocene in the presence of polyphosphoric acid or in the presence of anhydrous aluminium chloride using dichloromethane as the solvent to give 3- ferrocenyl isocoumarins. 7-Methoxy, 6-methyl, 5,7-dihydroxy, 6,7-dimethoxy and 5,7-dimethoxy derivatives of 3- ferrocenyl isocoumarin were synthesized. All the compounds were characterised by melting point determination, elemental and spectral analysis.
KEYWORDS:Ferrocene; Ferrocenyl Isocoumarins; Homophthalic Acid; Isocoumarin; Synthesis
Download this article as:| Copy the following to cite this article: Sinha N. Synthesis of 3- Ferrocenyl Isocoumarins. Orient J Chem 2020;36(5). |
| Copy the following to cite this URL: Sinha N. Synthesis of 3- Ferrocenyl Isocoumarins. Orient J Chem 2020;36(5). Available from: https://bit.ly/2HBPJK8 |
Introduction
Isocoumarin and its derivative have been found to exhibit interesting physiological properties. This is effective in central depressant activity, remedy for antimalerial and antiallergic1,2 retarding the clotting of blood3 ,tracheal and respiratory contractions4 ,as a strong pergative5,6 as anti-inflammatory7, antifungal8-10, antibacterial11 etc. Roger D. Berry has furnished a comprehensive review comprising isolation from natural sources, different method for synthesis, their properties, physiological activities and their biogenesis12. A review of literature revealed that 3- ferrocenyl isocoumarin were obtained from homophthalic anhydride and ferrocene in the presence of anhydrous aluminium chloride as dehydrating agen13-15. In the present work some of new ferrocynyl derivatives such as 7-methoxy, 6-methyl, 5,7-dihydroxy, 6,7- dimethoxy and 5,7- dimethoxy derivatives of 3- ferrocenyl isocoumarin were synthesized and characterized.
Experimental
All the chemicals used for the synthesis of derivatives of 3-ferrocenyl isocoumarin were AR grade of BDH (India) Ltd, Mumbai. Melting point was determined in soft glass capillaries in an electrothermal melting point apparatus and are uncorrected. IR spectra were recorded on Perkin Elmer 577 spectrophotometer using KBr pellets, 1H NMR spectra were recorded on Varian A-600 spectrophotometer in CDCl3 with TMS as an internal reference. Electronic spectra were recorded in ethanol on Perkin – Elmer 550 UV –visible Spectrophotometer.
The following steps were involved in the synthesis of the desired compounds :
Hydroxy benzoic acids were first converted into its methoxy derivatives by using dimethylsulphate and alkali. Hydroxy benzoic acids were prepared from aniline by usual method.
The derivatives of benzoic acid ( II a-e, 8.2 g), chloral hydrate (12.5g) and concentrated sulphuric acid ( 26ml ) were mixed together, shaken well and warm slightly to form a homogeneous solution and left at room temperature for 24 to 72 hours. It was then poured into crushed ice, the product (IIIa-e) separated was treated with sodium bicarbonate solution to remove unchanged acids, washed well with water and dried. It was crystallized from acetic acid or acetone in tiny prism.
The compound (III a-e, 10g) was dissolved in glacial acetic acid with slight warming . Zinc dust( 6.5g) was added in small portion during 1.5 hrs. The stirring was continued for an hour more after the complete addition of zinc dust. It was heated and filtered hot to remove zinc acetate. The filtrate was diluted with ice cold water. The solid separated was filtered, washed well with water and recrystellised from acetone (IV a-e), yield 3.4 g .
Substituted 2-( ββ – dichloroethyl) –benzoic acid (IV a-e, 7g) was added in small portion to preheated sulphuric acid (95%, 30ml).The resulting mixture was occasionally heated on water bath till no more hydrogen chloride gas was evolved.The addition of dichloro compound was so adjusted that the first lot had dissolved completely. It was poured over crushed ice, solid separated was filtered, washed and crystallized from ethanol, yield 2.1 g.
Homophthalic acid derivatives obtained (Va-e, 2.5g) were refluxed with acetic anhydride (7.5ml) for about 2 hours. The excess of acetic anhydride was distilled off and the remaining product was spread over watch glass and dried in hot air oven at 60 to 700 C.
In the last step the substituted homophthalic anhydride(VI a-e, 2.0g), ferrocene ( 2.0g) and polyphosphoric acid (12ml ) were heated in a glycerol bath at 140-150 0 C for about 2 hours. The mixture was well stirred after every few minutes. After the completion of reaction, the solution was poured over crushed ice and left overnight. The solid mass separated was filtered, washed well with water and treated with sodium bicarbonate solution to remove any unreacted homophthalic acid. It was filtered, washed and dried. The solid was then refluxed with petroleum ether (60-800 C) and filtered hot to remove any unreacted ferrocene. The solid left was crystallized from chloroform and petroleum ether (2:1) yield – 0.6g.
In another method, anhydrous aluminium chloride (2.5g) was suspended in dichloromethane (30ml) in a flask fitted with a separating funnel. It was cooled in ice. Ferrocene (2.2 g) and derivative of homophthalic anhydride (2.0g) was separately dissolved in minimum quantity of dichloromethane and taken together in the separating funnel which was fitted with a calcium chloride guard tube. The mixed solution was slowly added to the flask with thorough shaking. After the addition was complete, it was left overnight. The reaction mixture was refluxed for 2hours and poured over crushed ice. This was acidified with concentrated hydrochloric acid. It was extracted with chloroform.The chloroform later was recovered and the solid left was refluxed with petroleum ether (60-80 0C) for 2 hours to remove any unreacted ferrocene. The solid left was crystallized from chloroform and petroleum ether (2:1) to get desired product (VIIIa-e).
Results and Discussion
As mentioned above derivatives of 3-ferrocenyl isocoumarin were synthesized by the condensation of homophthalic anhydrides with ferrocene using anhydrous aluminium chloride or polyphosphoric acid as cyclising agent. The substituted homophthalic acids were prepared by condensing substituted benzoic acid with chloral hydrate, the product obtained were reduced with zinc and glacial acetic acid to furnish the respective 2- (ββ – dichloethyl) benzoic acid derivatives. The dichloro compound was further hydrolysed and simultaneously oxidized to the corresponding homothphalic acids by treating with concentrated sulphuric acid. These on further dehydration and condensation with ferrocene in the presence of phosphoric acid or anhydrous aluminium chloride using dichloromethane as solvent, 3- ferrocenyl isocoumarin derivatives were synthesised.
The chemical reactions involved in the synthesis of 3-ferrocenyl derivatives of isocoumarin are represented below :
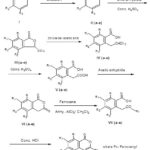 |
Figure 1 |
VIIIa – R, R2, R3 = H; R1 = -OCH3
VIIIb – R, R1, R3 = H; R2 = -CH3
VIIIc – R, R2 = H; R1,R3 = -OH
VIIId – R, R3 = H; R1, R2 = -OCH3
VIIIe – R, R2 = H; R1,R3 = -OCH3
The synthesized compounds were characterized by determination of melting point, elemental and spectral analyses. The IR spectra of 3-ferrocenyl derivatives of isocoumarin showed the absorption peak at 1610 cm-1 (-C=C-), 1735 cm-1 (C=O), 2855cm-1 (-CH stretching) and 3450 cm-1 (-OH) group. The UV absorption spectra of 3-ferrocenyl isocoumarin derivatives showed two peaks at 248nm and 324nm, characteristics of isocoumarin ring system. The NMR spectrum of compounds indicated absorption peaks at δ 7.2-7.5 (aromatic proton), 4.2-4.3 (9H, -C10H9Fe), 3.7-3.8(3H, -OCH3) and 1.2-1.3 (3H, -CH3). The results are presented below:
7-Methoxy – 3 –ferrocenyl isocoumarin (VIIIa)
(m. p.- Decomposes at 1400C);Found C – 66.21 %, H – 4.56 %; C20H16O3Fe requires C -66.69%, H – 4.44 %; UV (EtOH) λmax(nm) 255, 324; IR (KBr) (cm-1) : 2940, 2854 (-CH), 1735(C=O), 1610 (C=C), 1475 , 1425, 1110cm; 1H NMR (CDCl3)δ : 3.60 (3H,s,-OCH3,C7), 4.30 (9H, s, C10H9Fe, C3), 7.2-7.5( br, m, aromatic).
6- Methyl – 3 –ferrocenyl isocoumarin
(m.p.1420C);Found C – 69.12%, H – 4.78%; C20H16O2Fe requires C -69.79%, H – 4.65; UV (EtOH) λmax (nm) 245, 320; IR (KBr) (cm-1) : 2924 , 2842 (-CH stretching), 1725 (C=O), 1655, 1610(C=C), 1410 , 1120 ; 1H NMR (CDCl3) δ : 1.30 ( 3H,m,-CH3,C6), 4.20 ( 9H,s, -C10H9Fe, C3), 7.4-7.6( br, m, aromatic).
5,7-Dihydroxy –3-ferrocenyl isocoumarin
(m.p. 201-2030C); Found C – 62.86%, H – 3.46%; C19H14O4Fe requires C -63.0%, H – 3.86%; UV (EtOH) λmax( nm) 248, 324 ; IR (KBr) (cm-1) : 3450 ( br, -OH), 2920 , 2840 (-CH stretching), 1735(C=O), 1670, 1620 (C=C), 1450 , 1120 ; 1H NMR (CDCl3) δ : 4.20 ( 9H,s, -C10H9Fe, C3), 7.4-7.6 ( br, m, aromatic).
6,7- Dimethoxy -3-ferrocenyl isocoumarin
(m.p. 167-1690C);Found C – 64.11 %, H – 4.34 %;C21H18O4Fe requires C -64.63 0%, H – 4.61%;UV (EtOH) λmax (nm) – 244 , 322; IR(KBr) (cm-1) : 2940, 2820 (-CH stretching), 1785(C=O), 1665, 1620 (C=C), 1460, 1145;1H NMR (CDCl3) δ : 3.85 ( 6H,s, -OCH3, C6,C7) ,4.20 ( 9H, s, -C10H9Fe,C3), 7.25( br, m, aromatic).
5,7- Dimethoxy -3-ferrocenyl isocoumarin
(m.p. 201-2030C);Found C – 64.11 %, H – 4.34 %; C21H18O4Fe requires C -64.63 0%, H – 4.61% UV (EtOH) λmax (nm) 244, 322; IR (KBr) (cm-1) : 2940, 2820(-CH stretching), 1785(C=O), 1665, 1620 (C=C), 1460, 1145 ; 1H NMR (CDCl3) δ : 3.85 ( 6H,s, -OCH3, C6,C7), 4.20 ( 9H, s, -C10H9Fe,C3), 7.25 (br, m, aromatic).
Conclusions
7-Methoxy, 6-methyl, 5,7-dihydroxy, 6,7- dimethoxy and 5,7- dimethoxy derivatives of 3- ferrocenyl isocoumarin were synthesized. All the compounds were characterised by melting point determination, elemental and spectral analysis. The condensation of homophthalic anhydride with ferrocene in presence of anhydrous aluminium chloride and dichloromethane gave better result than the condensation of homophthalic anhydride in presence of polyphosphoric acid. It was found that substituted homophthalic acids were not condensing with ferrocene and compounds were recovered back. Hence homophthalic acids were first converted into corresponding anhydride by refluxing with acetic anhydride which was followed by condensation with ferrocene.
Acknowledgements
The author wish to thank Late Former Professor A. Ghosh for necessary guidance in the project and Dr. R. Sinha, Former Professors, T.M. Bhagalpur University for their support and discussion in the work. The support in spectral analysis carried out at BHU, Varanasi and the financial support from UGC is gratefully acknowledged. The author is thankful to the Principal, Jamshedpur Cooperative College, Jamshedpur for his encouragement.
References
- Chinworrungsee, M; J. Chem.Soc; 2002, 1, 2473.
CrossRef - Matsuda, H; Shimoda, H; Yoshikawa, M; Bioorg. Med. Chem.Lett, 1999, 7, 1445.
CrossRef - Pal, Sabarbani; Chatare , Vijay Kumar; Pal, Manojit ; Current Org Chemistry, 2011, 15, 782.
CrossRef - Yamatodani,S; Nippon Nogeikagaku Kaishi,1963 , 37, 240 .
- Schmidt, L; Seeger, E; Chem.Abstr ;1956, 12,308.
- Buu-Hoi ,M; Compt rend, 1940, 210, 418 .
- Wakabayashi, Toshio; Watanabe, Kenzo( Japan); Kokai Tokkyo Koho, 1978, 78, 879.
- Wakabayashi, Toshio; Watanabe, Kenzo( Japan); Kokai Tokkyo Koho, 1979, 79, 486 .
- Nakajima ,S; Sugiyama, S; Suto, M; Chem Abstr, 1979, 91,39262r.
- Yadav ,Poonam ; Purohit, Nalini V; Der Pharma Chemica, 2011, 3, 189.
- Mues, Voker; Behrenz, Wolfgang; Eur. Pat. Appl., 1980, 4, 929.
- Berry, Roger D; Chemical Review, 1964, 64, 230.
CrossRef - Boichard, J. (1961) Compt rend. 253, 2702.
- Desai, H.K; Usgaonkar, R.N; Jour.Ind.Chem.Soc; 1963, 40, 239.
- Sumani, Jimmy Yaphet Yaphet; URI- https://hdl.hardel.net/10539/26737, 1980.

This work is licensed under a Creative Commons Attribution 4.0 International License.










