Synthesis, Characterization and Cytotoxicity of Ni (II), Pd (II), Pt (II) Complexes with 6-Methoxy-2, 3, 4, 5-Tetrahydropyridine (MTP)
Hikmat Ali Mohamad1* , Widad Taha Al-Kattan2, Zena Mashal Al-Daly2 and Ali Nafia Najim2
, Widad Taha Al-Kattan2, Zena Mashal Al-Daly2 and Ali Nafia Najim2
1College of Education, University of Salahaddin, Erbil, Iraq.
2College of Science, University of Mosul, Mosul, Iraq.
Corresponding Author E-mail: hikmatmohama@yahoo.com
DOI : http://dx.doi.org/10.13005/ojc/360515
Article Received on : 21-07-2020
Article Accepted on : 31-08-2020
Reaction of 2 moles of (MTP) with one mole of MCl2 gave colored complexes with general formula; [M(MTP)2Cl2]. [M = Ni+2, Pd+2, and Pt+2]. All synthesized complexes are well-characterized using, FT-IR, 1HNMR, 13NMR, UV-Vis. Spectra furthermore of magnetic susceptibility, CHN analysis, and molar conductivity. The results illustrated that divalent metal ions were coordinated with the ligands through nitrogen atoms in square planar spatial arrangements. The complexes were screened for their cytotoxicity effects versus MCF-7 cell line and showed that [Pt(MTP)2Cl2] complex has good cytotoxicity in comparison with the other complexes.
KEYWORDS:Cisplatin; Cytotoxicity; MCF-7 Cell Line; Metal Complexes
Download this article as:| Copy the following to cite this article: Mohamad H. A, Al-Kattan W. T, Al-Daly Z. M, Najim A. N. Synthesis, Characterization and Cytotoxicity of Ni (II), Pd (II), Pt (II) Complexes with 6-Methoxy-2, 3, 4, 5-Tetrahydropyridine (MTP). Orient J Chem 2020;36(5). |
| Copy the following to cite this URL: Mohamad H. A, Al-Kattan W. T, Al-Daly Z. M, Najim A. N. Synthesis, Characterization and Cytotoxicity of Ni (II), Pd (II), Pt (II) Complexes with 6-Methoxy-2, 3, 4, 5-Tetrahydropyridine (MTP). Orient J Chem 2020;36(5). Available from: https://bit.ly/37YHJOl |
Introduction
N-heterocyclic (NHC) compounds constitute a new type of ligands in coordination chemistry and organometallic chemistry1, they belong to a new family of ligands with electronic characteristics similar to those of the phosphines2. The ligands of heterocyclic rings contain nitrogen atom as a donor set the have π-acidity properties and they form many color complexes with transition elements in various oxidation state3. Recently mixed ligand of dithizone metal ion complexes were reported to have anticancer activity in vitro4. The current work reports synthesis of new complexes contains nitrogenic ligand of general structure [M(MTP)2Cl2] and their characterizations as tetra coordination number as square planar geometry. Also, the study of their anticancer ability versus MCF-7 cell line.
Experimental
Materials and Instrumentation
All chemicals used in this work are in of reagent grade and used without farther purification as supplied from Sigma, Solarbio, Fluka, Scharlue, BDH, Capricorn, Santacruze Biotechnology, and Bio-world. Electro molar conductivities for the 10-3 Msolution of the synthesized complexes in (DMSO) using [Senz µSiemen tester 4200] college of education / Salahaddin University /Erbil. Elemental analysis (C.H.N) was measured using [EuroEA 3000/Italy] in the service laboratory at Ibn al-Haitham /college of education / University of Baghdad. FT-IR spectra were recorded using [Ftir-600 FTIR Spectrometer Biotech Engineering Management.UK] in the service laboratory at Ibn al-Haitham college of education/University of Baghdad. The UV/Vis were recorded using, AE-UV1609 Spectrometer (UK) Co.] in the college of education/Salahuddin University/Erbil. 1HNMR and 13CNMR spectra were recorded on Brucker 300MHZ with tetramethylsilane as an internal standard in DMSO-d6 Measurements were made at water, environment and arid regions research center/ Al- a Bayt University/ Jorden.
Synthesis of [M(MTP)2Cl2]
To a methanol (25 ml) solution of (MTP) (0.23g, 2mmol), an equivalent methanol (25 ml) solution of NiCl2.6H2O (0.24g, 1mmol), PdCl2 (0.18g, 1mmol) and K2PtCl4 (0.42, 1mmol) was added in dropwise. Thereafter, the mixture was stirred for 9-14 h at room temperature. The resultant solution was filtered and set aside for a few days to give colored precipitates. The precipitates were filtered off and dried under vacuum.
Cytotoxicity Assay
Cell Lines
MCF-7 cells were provided from the Iraqi Cell Bank and kept and treated as in5
Cytotoxicity Assay
To emphasize the effect of cytotoxicity, methyl thiazolyl tetrazolium (MTT)5 viability test was measured using 96-well plates. MCF-7 were sowed at 1 × 104 cells/well. Monolayer achievement has been occurred after 24 h. The cell viability assay has been taken after 72 h by addition 28 µL of 2 mg/mL of MTT solution, the cells were incubated for 1.5 h at 37 °C. The crystals which formed on the wells, during the incubating were dissolved by the addition of 130 µL of DMSO6. Absorbances were recorded at 492 nm. All tests were performed in triplicate and he inhibition rate of cell growth was obtained as follows:
Inhibition growth rate = A-B/A x 100 (where A and B are absorbances of control and tested compounds respectively).
Results and Discussion
A synthesized divalent metal complexes (Scheme 1) were obtained from reaction of Ni (II), Pd (II) and Pt (II) chloride salts with (MTP) in (2:1) ratio produce neutral-colored complexes. All complexes have good solubility in dimethylsulphoxide. The conductivities in 10-3M dimethylsulphoxide solutions are between (10-20) Ω-1 cm2 mol-1 7. This is inconsistent with the stoichiometry suggested complexes. The analytical data of synthesized complexes are shown in the (Table 1), were agreement with the calculated values. The suggested molecular formulas were also supported by spectral and magnetic moment measurements.
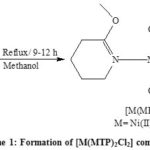 |
Scheme 1: Formation of [M(MTP)2Cl2] complexes |
Magnetic Susceptibility
The magnetic moments were taking at 250C. The results proved square planar structures for all complexes 8
Table 1: Physical analytical data for synthesized complexes
|
Elemental analysis, calc. (found) % |
M% |
Yield %
|
Melting point |
Color |
µeff |
Complexes |
No. |
|||
|
N |
H |
C |
||||||||
|
7.63 (7.87) |
6.19 (6.23) |
40.25 (40.50) |
15.68 (16.49) |
72 |
– |
Blue- green |
Dia. |
[Ni(MTP)2Cl2] |
1 |
|
|
6.74 (6.94) |
5.49 (5.47) |
35.13 (35.71) |
26.10 (26.37) |
77 |
143-148 |
Yellow-orang |
Dia. |
[Pd(MTP)2Cl2] |
2 |
|
|
5.66 (5.69) |
4.26 (4.50) |
28.36 (29.28) |
38.11 (39.63) |
68 |
– |
Dark Brown- |
Dia. |
[Pt(MTP)2Cl2] |
3 |
|
Electronic Transitions Spectra
The electronic transition spectra of the ligand and its synthesized complexes in the 10-3 M (DMSO) solution were illustrated in (Table 2). The electronic transition band at 37450 cm-1 is for π- π* transition within ligands. A given spins allowed transitions at (21978) cm-1 (υ1), (22883-24875) cm-1 (υ2), (26109-28571) cm-1 (υ3) were assigned to 1A1g→1A2g (υ1), 1A1g→1B1g (υ2) and 1A1g→1Eg (υ3) transitions respectively. The strong bands at (29850-31847) cm-1 are due to (MLCT) and d→d transitions. The transition band at 32786- 36363 cm-1 is due to the n→p* transitions (LMCT) of the pyridine ring. These transitions value is indicated to square planar geometry 9-11.
Table 2: UV/Vis transition for the synthesized complexes
|
|
Transitions |
Absorption band |
Complexes |
No. |
|
|
nm |
cm-1 |
||||
|
Sp. |
1A1g ® 1B1g 1A1g ® 1Eg C.T |
437 350 314 |
22883 28571 31847 |
[Ni(MTP)2Cl2] |
1 |
|
Sp. |
1A1g ® 1B1g 1A1g ® 1Eg C.T |
402 383 305 |
24875 26109 32786 |
[Pd(MTP)2Cl2] |
2 |
|
Sp. |
1A1g ® 1A2g 1A1g ® 1B1g C.T C.T |
455 411 335 275 |
21978 24330 29850 36363 |
[Pt(MTP)2Cl2] |
3 |
Infrared Spectral
IR frequencies of the free ligand and complexes are tabulated in (Table 3). A peak at (3056) cm-1 is for υ(CH) stretching structure12, a frequency band at (2960) cm-1 which may be due to the υ(CH) of methoxy group, generally it has been observed at lower frequencies than the normal υ(CH) for (CH3-) group 13. The frequency band at 1625 cm-1 is related to υ(C-C) in the pyridine ring. A υ(C-O-C) is at 1260 Cm-1. A frequency band at 1480 cm-1 which are corresponding to υ(CN) stretching vibrations, all complexes showed a negative shift in ν(CN) confirmed the participate of nitrogen in coordination 14.
Table 3: IR band in cm-1 for the ligand and the complexes
|
Compound |
n(CH) ring |
n(CH) methoxy |
n(CN) |
n(CC) |
n(C-O-C) |
|
MTP |
3056 |
2960 |
1260 |
1625 |
1480 |
|
[Ni(MTP)2Cl2] |
3100 |
2962 |
1256 |
1655 |
1442 |
|
[Pd(MTP)2Cl2] |
3036 |
2954 |
1247 |
1666 |
1438 |
|
[Pt(MTP)2Cl2] |
3085 |
2955 |
1256 |
1630 |
1448 |
NMR Spectra
1H and 13C NMR bands were obtained using (DMSO-d6) as a solvent (Table 4) and (Figure 1-3). The spectra showed two sets of bands which attributed to the pyridine part and substituted aliphatic of the MTP ligand. The 1H NMR bands of coordinated ligand were shifted more than free ligand and they agree with complexion with metal ions through N of pyridine ring15. The 13C NMR data in all complexes show. No observed change in this signal in complexes formation and this due to uncoordinated aromatic carbon in the metal ion complexes16.
Table 4: NMR spectral for band in δ(ppm) for the ligand and their complexes
|
Compound |
Atom |
1HNMR |
Atom |
13CNMR |
|
MTP |
1H |
3.53 |
1C |
45.4 |
|
2H |
1.3 |
2C |
22.2 |
|
|
3H |
1.77 |
3C |
16.4 |
|
|
4H |
2.11 |
4C |
29.7 |
|
|
5H |
3.47 |
5C |
161.7 |
|
|
6C |
53.2 |
|||
|
[Ni(MTP)2Cl2] |
1H |
2.6 |
1C |
38.66 |
|
2H |
1.3 |
2C |
22.24 |
|
|
3H |
1.96 |
3C |
16.58 |
|
|
4H |
2.1 |
4C |
37.65 |
|
|
5H |
3.53 |
5C |
162.14 |
|
|
6C |
54.15 |
|||
|
[Pd(MTP)2Cl2] |
1H |
2.82 |
1C |
38.67 |
|
2H |
1.3 |
2C |
21.65 |
|
|
3H |
1.77 |
3C |
18.2 |
|
|
4H |
2.23 |
4C |
29.74 |
|
|
5H |
3.59 |
5C |
159.41 |
|
|
6C |
51.2 |
|||
|
[Pt(MTP)2Cl2] |
1H |
2.94 |
1C |
39.39 |
|
2H |
1.3 |
2C |
21.36 |
|
|
3H |
1.6 |
3C |
18.23 |
|
|
4H |
2.53 |
4C |
31.34 |
|
|
5H |
3.88 |
5C |
162.47 |
|
|
6C |
51.620 |
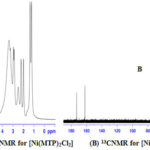 |
Figure 1: 1HNMR for [Ni(MTP)2Cl2] (A) and 13CNMR for [Ni(MTP)2Cl2](B) |
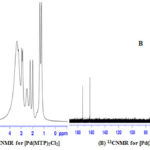 |
Figure 2: 1HNMR for [Pd(MTP)2Cl2] (A) and 13CNMR for [Pd(MTP)2Cl2] (B) |
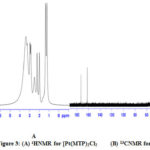 |
Figure 3: 1HNMR for [Pt(MTP)2Cl2 (A) and 13CNMR for [Pt(MTP)2Cl2] (B) |
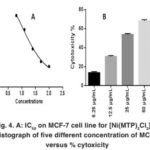 |
Figure 4: A: IC50 on MCF-7 cell line for [Ni(MTP)2Cl2] B: Histograph of five different concentration of MCF-7 versus % cytoxicity |
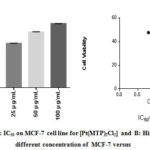 |
Figure 5: A: IC50 on MCF-7 cell line for [PT(mtp)2CI2] B: Histograph of five different concentration of MCF-7 versus % cytoxicity |
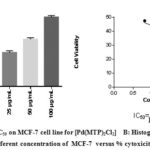 |
Figure 6: A: IC50 on MCF-7 cell line for [Pd(MTP)2Cl2] B: Histograph of five different concentration of MCF-7 versus % cytoxicity |
Cytotoxicity Results
Our study on synthesized Ni (II), Pd (II) and Pt (II) metal ion complexes were evaluated against human MCF-7 cells. Cisplatin was employed as in a reference [6]. It is found that the [ Pt (MTP)2Cl2] complex has the highest IC50 cell viability and p-value17. The results included (cell growth %) or (% of cell treatment). The low concentration kills about 19% of MCF-cell line (Table 5) and (Figure 4- 6). Whilst Ni (II), Pd (II) complexes were less active than cisplatin is in the micromolar range. On the basis of existence of methoxy moiety on both pyridine rings play important role in inhibition linking of N of DNA with central metal, thus leading to increase cytotoxicity meta lion from the complex 18.
Table 5: Cytotoxic abilities of the synthesized complexes on the MCF-7 cells
|
Sample |
Con. |
6.25 µg/mL |
12.5 µg/mL |
25 µg/mL |
50 µg/mL |
100 µg/mL |
|
Cisplatin |
Mean |
24.67 |
51.80 |
71.87 |
88.10 |
94.00 |
|
P value |
0.0343 |
0.0148 |
0.0100 |
0.0065 |
0.0068 |
|
|
Significant (alpha=0.05) |
Yes |
Yes |
Yes |
Yes |
Yes |
|
|
[Pt(MTP)2Cl2] |
Mean |
13.66 |
30.71 |
53.87 |
68.00 |
74.98 |
|
P value |
0.0024 |
0.0004 |
0.0001 |
0.0094 |
0.0001 |
|
|
Significant (alpha=0.05) |
Yes |
Yes |
Yes |
Yes |
Yes |
|
|
[Ni(MTP)2Cl2] |
Mean |
9.091 |
23.97 |
42.84 |
53.69 |
61.89 |
|
P value |
0.1318 |
0.0008 |
0.0024 |
0.0036 |
0.0011 |
|
|
Significant (alpha=0.05) |
No |
Yes |
Yes |
Yes |
Yes |
|
|
[Pd(MTP)2Cl2] |
Mean |
10.40 |
24.18 |
33.79 |
46.91 |
68.91 |
|
P value |
0.0367 |
0.0215 |
0.0228 |
0.0147 |
0.0101 |
|
|
Significant (alpha=0.05) |
Yes |
Yes |
Yes |
Yes |
Yes |
All complexes have been synthesized and characterized; the results indicate that all of them have square planer geometry in which the metal ions coordinate with the ligand through a nitrogen atom. The cytotoxicity effects study of the prepared complexes versus MCF-7 cell line in different concentrations showed that [Pt (MTP)2Cl2] has good cytotoxicity in comparison with the other complexes.
Acknowledgement
The authors are thankful to the Chemistry Department, College of Education/ Salahuddin University for assisting this work. The authors also thank the University of Mosul, College of Science for a partial help of this study.
Conflict of Interests
No conflict of interest.
References
- Zhang, X. Z.; Chen, X.; Fu, W.; Wang, D. Organometallics. 2007, 26, 6636-6642.
CrossRef - Herrmann, W.A.; Köcher, C. Chem. Int. Ed. 1997, 36, 2162-2187.
CrossRef - Cotton, F. A.; WilKinson, G. Advanced Inorganic Chemistry. 1980, 4th J. Wiley and Sons, New York, 129.
- Mohamad, H. A. Oriental Journal of Chemistry. 2018, 34, 1919-1925.
CrossRef - Al-Shammari, A.M.; Alshami, M.A.; Umran, M.A.; Almukhtar, A.A.; Yaseen, N.Y.; Raad, K.; Hussien, A.A. Breast Cancer: Targets and Therapy. 2015, 7, 223-230.
CrossRef - Al-Shammari, A.M.; Salman, M.I.; Saihood, Y.D.; Yaseen, N.Y.; Raed, K.; Shaker, H.K.; Ahmed, A.; Khalid, A.; Duiach, A. Biomedicines. 2016, 4, 3-10.
CrossRef - Ali, K.O.; Mohammad, H.A. J. P. A. S. 2020, 32, 15-23.
CrossRef - Cotton, F.; Wikinson, G. Advanced Inorganic chemistry. 1999. 6th, John Wiley and Sons, New York, 851-866
- Kovala-Demertzi, D.; Domopoulou, A.; Demertzis, M.A.; Valle, G; Papageorg iou, A. Inorg. Biochem. 1997, 68, 147-155.
CrossRef - Papageorgiou, A.; Iakovidou, Z.; Mourelatos, D.; Mioglou, E.; Boutis, L.; Kotsis, A.; Kovala-Demertzi, D.; Domopoulou, A.; West, D.X.; Dermetzis, M.A. Anticancer Res. 1997, 17, 247-251
- Goldin, A.; Sofina, Z.; Syrkin, A. National Cancer Inst Monograph. 1980 USA, No 55.
- Muthua, S.; Paulrajb, E.I. Chem. Pharm. Res. 2011, 5, 323-339
- Karabacak, M.; Sinha, L.; Prasad, O.; Asiri, A.M.; Cinar, M.; Shukla, V.K. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2014, 123, 352-362.
CrossRef - Wandas, M.; Puszko, A. Heterocycl. Compd. 2000, 36, 796-800.
CrossRef - Debono, N.; Iglesias, A.M.; Sanchez, F. Synth. Catal. 2007, 349, 2470-2476.
CrossRef - Abel, E.W.; Ahmed, A.S; Farrow, G.W.; Orrell, K.G.; Šik, V. Chem. Soc. Dalton Trans. 1977, 1, 47-52.
CrossRef - Eren, G.; GÜMÜŞ, F.; YILMAZ, Ş. Fabad J Pharm Sci. 2011, 36, 69-73
- Mohamed, H.A.; Lake, B.R.; Laing, T.; Phillips, R.M.; Willans, C.E. Dalton Trans. 2015, 44, 7563-7569.
CrossRef

This work is licensed under a Creative Commons Attribution 4.0 International License.









