Molecular Interactions and Aggregation Behavior of Cloxacillin Sodium in Water and Aqueous NaCl Solutions through Volumetric and Ultrasonic Sound Velocity Studies at 298.15 K
Mst. Roksana Khatun1* , Md. Monirul Islam2 and Md. Din Islam1
, Md. Monirul Islam2 and Md. Din Islam1
1Department of Chemistry, Faculty of Engineering & Technology. Chittagong University of Engineering & Technology, Chattogram-4349, Bangladesh.
2Department of Chemistry, University of Rajshahi, Rajshahi-6205, Bangladesh.
Corresponding Author E-mail: ark19mrk@gmail.com
DOI : http://dx.doi.org/10.13005/ojc/360510
Article Received on : 28-06-2020
Article Accepted on : 02-10-2020
Molecular interactions and aggregation behavior of cloxacillin sodium (CloNa) in water and aqueous NaCl solutions was studied through volumetric and ultrasonic sound velocity method at 298.15 K and atmospheric pressure. Density, ρ, and ultrasonic sound velocity, u, of cloxacillin sodium in water and aqueous NaCl solutions were measured with a digital density and ultrasonic sound velocity analyzer (DSA 5000, Anton Paar, Austria). And from the investigational data, apparent molar volume,〖 ϕ〗_v, adiabatic compressibility, βS, apparent molar adiabatic compressibility, ϕ_k, limiting apparent molar volume, ϕ_v^0, limiting apparent molar adiabatic compressibility,〖 ϕ〗_k^0 , and aggregation concentration, CMC of cloxacillin sodium was calculated. The outcomes indication exhibits very significant evidence about the interactions among solute-solvent-co-solute (solute-solute, solute-solvent) and aggregation behavior in the aqueous environment and this result would be useful for the drug action in human body with pharmacological applications.
KEYWORDS:Adiabatic Compressibility; Aggregation; Apparent Molar Adiabatic Compressibility; Apparent Molar Volume; Cloxacillin Sodium; Nacl; Solute-Solute Interactions; Solute-Solvent
Download this article as:| Copy the following to cite this article: Khatun R. M, Islam M. M, Islam M. D. Molecular Interactions and Aggregation Behavior of Cloxacillin Sodium in Water and Aqueous NaCl Solutions through Volumetric and Ultrasonic Sound Velocity Studies at 298.15 K. Orient J Chem 2020;36(5). |
| Copy the following to cite this URL: Khatun R. M, Islam M. M, Islam M. D. Molecular Interactions and Aggregation Behavior of Cloxacillin Sodium in Water and Aqueous NaCl Solutions through Volumetric and Ultrasonic Sound Velocity Studies at 298.15 K. Orient J Chem 2020;36(5). Available from: https://bit.ly/3ohmnRJ |
Introduction
In pharmacokinetics, drug is a very essential chemical material that used for the treatment of various disease such as therapy, prevention /diagnosis, develop mental well-being 1 etc. To recognize the drug action in human body at the molecular level, the physicochemical properties of drugs are very important2. By studying the physicochemical properties the binding possibility of biologically active molecules in presence of several solvents or co-solutes can be well examine3.
Cloxacillin sodium (CloNa) is a sodium salt of cloxaclliin and semi synthetic beta-lactamase penicillin antibiotic having remarkable antibacterial actions. Cloxacillin Sodium (CloNa) shows4–6 noticeable vulnerability to acid and base reagents, several nucleophiles, oxidizing agents, metal ions, and many solvents and is broadly used in the treatment of blood poisoning, parenchyma of skin infection, pneumonia, etc.7.
Volumetric2,8–12 and ultrasonic sound velocity13,14 technique is very convenient and reliable tool to well investigation in case of drugs, amino acids, polymers etc analysis. Thermodynamic properties such as, , and aggregation concentration of any solution are very crucial parameter to recognize the solute–solvent and solute–solute interactions15.
For cloxacillin Sodium (CloNa), there are some reports on the stability of solid CloNa, degradation16, interactions with protein17, Taboada18 examined aggregation behavior of CloNa in aqueous and aqueous NaCl with light-scattering, NMR techniques. However, there is still a lack of knowledge about the interactions among solute-solvent-co-solute and aggregation behavior of CloNa in water or electrolyte solvents. In this background, this work presents a study on the solute-solvent, solute-solute interactions and aggregation behavior or properties of cloxacillin sodium (CloNa) in water and aqueous NaCl solutions through volumetric and ultrasonic velocity method. In this study, NaCl (co-solute) is taken because it is the chief electrolyte in the extracellular fluid19 in human body rather than other ions, such as Ca+ , K+ , Mg+ , HPO4−, and HCO3−. The range of Na+ in human body is ∼92 % in case of over-all positive ions and Cl− is ∼68 % for negative ions.
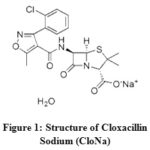 |
Figure 1: Structure of Cloxacillin Sodium (CloNa) |
To understand the complete interactions of CloNa with electrolytes, density, ρ, and ultrasonic sound velocity, u, of CloNa in water and NaCl aqueous solutions was measured at 298.15 K and atmospheric pressure. From density and ultrasonic sound velocity data, apparent molar volumes,, adiabatic compressibility, βS, apparent molar adiabatic compressibility, , their parallel limiting parameters, and aggregation concentrations (CMC) were calculated. The tremendous results are represented in our paper.
Experimental Details
Materials
CloNa and sodium chloride were used in this study and collected from Beximco Pharmaceuticals Limited (Bangladesh), and Loba Chemie Pvt. Ltd. (India) respectively. The chemicals specifications are given below in Table 1 that used in this investigation.
Table 1: Chemicals Specifications
|
Chemical title |
Molar mass/(kg·mol−1) |
Clarity declared by provider |
Origin |
|
Cloxacillin sodium(CloNa) |
0.457864 |
mass fraction, ≥ 0.990 |
Beximco Pharmaceuticals Limited (Bangladesh) |
|
NaCl |
0.058442 |
mass fraction, ≥ 0.995 |
Loba Chemie Pvt. Ltd(India) |
Density and Ultrasonic Sound Velocity Measurement
Each solution prepared instantly with highly purified water. Because water was redistilled and degassed with specific conductance of < 10−6 S (cm−1). By weighing, solution was prepared in molality using a balance (B204-S, Mettler Toledo, Switzerland) having a precision of ± 0.0001 g. Molality uncertainty for solutions is up to ± 2·10−5(mol kg−1) . An analyzer (DSA 5000, Anton Paar, Austria) was used to examine density, ρ, and ultrasonic sound velocity, u, of the solutions. A density check carried out at 20 °C with a) distilled and degassed water, b) dry air. For each measurement, the density meter calibrated with redistilled and gas free water in the investigational temperature area. Temperature sensitivity was controlled by a built-in Peltier method to ± 1·10−3 K. The instrument sensitivity for density with respect to an accuracy is 1·10−3(kg m−3) and for ultrasonic sound velocity 1·10−2(m s−1). The density and ultrasonic sound velocity uncertainty was obtained ± 5·10−3 kg m−3 & ± 5·10−2 m s−1, respectively.
Results and Discussion
The measured density ρ & ultrasonic sound velocity u of CloNa (as a function of molality) in water and aqueous sodium chloride solution at different concentration are taken in Table 2. The apparent molar volume,, adiabatic compressibility, βS and apparent molar adiabatic compressibility, calculated from measured density ρ and ultrasonic sound velocity u using equation (1), (2) & (3) are shown in Table 2.
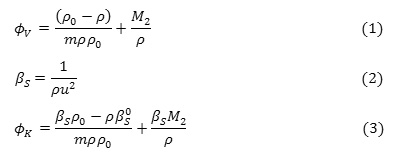
where,
m = molality, ρ = density of solution,= adiabatic compressibility, ρ0 = density of solvent,= adiabatic compressibility and M2 = molar mass
The reported standard combined uncertainties in m, , , , and CMC were calculated based on first order Taylor series approximation with the help of their equivalent error equations of the following type,
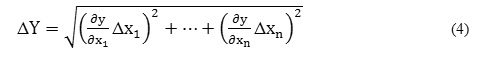
where,
Δx1, Δx2…Δxn= standard errors,
x1, x2… xn =independent variables
The changes of apparent molar volume, and apparent molar adiabatic compressibilities, of CloNa with respect to molality at 298.15K are shown in Figure 2(a) and 2(b). The values of, are increased with respect to CloNa concentration, indicate the strong solute-solute interaction. At a certain point the increasing rate is changed and shows the break point. To investigate the properties i.e. solute-solvent, solute-solute interaction and aggregation behavior,, and CMC are very useful and these parameters can be obtained by regression of and vs molality data to piece-wise linear model of the following type :
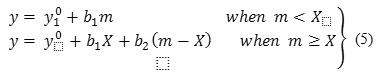
where,
y =or,
m = molality,
X = CMC,
y0 = limiting parameter at infinite dilution or,
b= slopes Sv and Sk,
Subscripts 1 = for pre-CMC region,
Subscripts 2= for post-CMC region,
Fitting parameters with their standard uncertainties are presented in Table 3.
 |
Figure 2: The variation of (a)and (b) values of CloNa in m = (■- 0.000, ■- 0.10049,■- 0.0586; ■-0.30012,■-0.40623,■-0.50572, ■-0.60480) M aqueous NaCl solutions at 298.15 K. Click here to View figure |
Solute-Solvent Interactions
The calculated, &indicates the interactions between solute and solvent molecules (solute-solvent). The changes of of CloNa with respect to NaCl molality at various temperatures are shown in Figure 3(c). The values of&are goes to minima with the concentration of sodium chloride that is sign for the presence of strong interactions among solute, solvent and co-solute in the mixtures.
According to the configuration of CloNa (Figure. 1), the probable interactions of CloNa with NaCl are(a) two types ion−ion interactions such as i) COO−(CloNa) with Na+(NaCl) ii) Na+ (CloNa) with Cl− (NaCl), (b) Ion−hydrophilic interactions of Na+(NaCl) with the hydrophilic groups (O, F, N, S and Cl) of CloNa, and(c) Ion−hydrophobic interactions of Na+(NaCl) with the hydrophobic groups (alkyl groups, benzene ring) of CloNa. The co-sphere overlap model20 recommends that (a) and (b) type interactions gives positive contribution to , however type (c) gives an opposite contribution to i.e. negative. The decrease ofvalues with respect to increase in NaCl concentration suggests that at the beginning the ion− hydrophobic interaction exceeds over the ion− hydrophilic and ion−ion. After that ion− hydrophilic, ion−ion interaction exceeds owing to the increasing interactions with excess co-solute (NaCl) molecules. The variations of of CloNa against the concentration of NaCl are shown in Figure 3(d). The values are more negative at the minima with the rising of NaCl concentration, indicates water clustering occurrences by the increasing of hydrogen bond. Allowing the Frank and Wen21 model, around the ions (low-charge density) water molecules are randomly distributed and bulk water molecules arranged orderly. The water molecules are more compressible as a monomeric form. The O, F, N, S and Cl (hydrophilic groups) of FluNa, have weak partial charges and (b) type interaction gives monomeric water molecules to bulk due to relaxation, where they form cluster water molecules and gives a negative role to. This is also supported well-accommodated aromatic rings22 in electrolyte solution instead of pure water. After minima, the magnitude of increase i.e, compressibility increases due to dominate (a) type interactions.
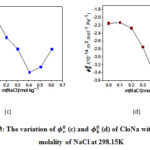 |
Figure 3: The variation of (c) and (d) of CloNa with respect to molality of NaCl at 298.15K Click here to View figure |
Solute-Solute Interactions
The interactions of solute with solute (solute-solute) in terms of slope Sv and Sk are displayed in Figure 4 with respect to NaCl molality at 298.15 K. The slopes Sv1, Sk1 (pre- CMC) and Sv2, Sk2 (post-CMC) goes to the maxima. It is clearly declaring that Sv1, Sk1 provide information about the interactions between CloNa molecules, while Sv2, Sk2 deliver about the micelle CloNa interactions. The Sv1, Sk1, Sv2 and Sk2 values show strong interactions among the CloNa-CloNa molecules and micelle- micelle. And increasing trend with the NaCl concentration may be due to decreasing electrostatic repulsion among their head group charge. But for decreasing trend, the exact interpretation is difficult. However, in case of pre-CMC slopes there may be increase electrostatic repulsion among their head group charge due to excess co-solute (NaCl) molecule interaction and for post-CMC slopes transition happen from spherical micelles to other geometrical shapes23-25.
 |
Figure 4: The variation of slopes of CloNa with respect to NaCl molality at 298.15K. The figure (e), (f) represent pre-CMC and (g), (h) represent post-CMC slopes. The presented slopes are achieved from and Click here to View figure |
Aggregation Behaviors
The piecewise linear regression model is used for determining CMC point as shown in equation 5. X is the critical micelle concentration. The variation of CMC values of CloNa are presented in Figure 5 with respect to NaCl molality. Previously CMC values reported by light scattering18,26 and NMR18 techniques in water and aqueous NaCl solutions exhibits a near agreement with these CMC values. It is observed from Figure 5(i) and 5(j) that the CMC values attained from – m, – m data show similar action to NaCl concentration. The gradually decreasing CMC values acquire a minimum value and then increasing with the increasing NaCl concentration. The decreasing trend is owing to the decreasing of electrostatic repulsion between the polar head groups (O, F, N, S and Cl) in presence of NaCl. And after that at the higher concentration of NaCl, the transition of spherical micelle25 to cylindrical micelle is responsible for increasing CMC values. Because at higher salt concentrations, solute molecules are packed compactly and they successfully adopt a more cylindrical shape and cylindrical shape contain additional solute molecules rather than spherical that is form at upper solute concentrations.
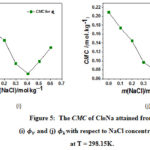 |
Figure 5: The CMC of CloNa attained from (i) and (j) with respect to NaCl concentration at T = 298.15K. |
Table 2: The Density, ρ, and Ultrasonic Sound Velocity, u, Apparent Molar Volume, ϕv , Adiabatic compressibility, βs , Apparent molar Adiabatic compressibility, ϕk of CloNa in Water and Aqueous NaCl Solutions at 298.15 K and Atmospheric Pressurea.
|
m/mol·kg-1 |
ρ /kg m−3 |
u /m s−1 |
ϕv /10−6 m3 mol−1 |
βs/10-10. Pa-1 |
ϕk /10−14 m3 mol−1 Pa−1 |
|
|
Cloxa+water |
||||
|
0.00000 |
994.069 |
1519.98 |
– |
4.3542±0.0006 |
– |
|
0.03709 |
999.780 |
1525.15 |
303.10±0.20 |
4.3000±0.0006 |
-1.70±0.20 |
|
0.07391 |
1005.293 |
1529.84 |
303.49±0.08 |
4.2503±0.0006 |
-1.20±0.10 |
|
0.12465 |
1012.670 |
1535.36 |
303.90±0.06 |
4.1890±0.0005 |
-0.60±0.06 |
|
0.16916 |
1018.936 |
1539.36 |
304.22±0.04 |
4.1416±0.0005 |
-0.04±0.05 |
|
0.20779 |
1024.205 |
1542.20 |
304.60±0.04 |
4.1052±0.0005 |
0.45±0.04 |
|
0.22741 |
1026.844 |
1543.90 |
304.70±0.03 |
4.0856±0.0005 |
0.57±0.04 |
|
0.27564 |
1033.193 |
1547.89 |
304.96±0.00 |
0.0000±0.0000 |
0.84±0.00 |
|
0.33466 |
1040.711 |
1552.10 |
305.23±0.02 |
3.9887±0.0005 |
1.19±0.02 |
|
0.36240 |
1044.138 |
1553.97 |
305.40±0.02 |
3.9660±0.0005 |
1.34±0.02 |
|
|
Cloxa+water+NaCl(0.10049m) |
||||
|
0.00000 |
998.077 |
1525.87 |
– |
4.3033±0.0006 |
– |
|
0.04265 |
1004.609 |
1531.81 |
303.00±0.20 |
4.2422±0.0006 |
-1.50±0.20 |
|
0.09145 |
1011.840 |
1537.39 |
303.50±0.10 |
4.1814±0.0005 |
-0.67±0.09 |
|
0.12129 |
1016.131 |
1540.28 |
303.83±0.07 |
4.1481±0.0005 |
-0.22±0.07 |
|
0.16344 |
1022.045 |
1543.73 |
304.23±0.05 |
4.1057±0.0005 |
0.38±0.05 |
|
0.20903 |
1028.260 |
1547.37 |
304.59±0.04 |
4.0617±0.0005 |
0.79±0.04 |
|
0.25362 |
1034.172 |
1550.95 |
304.85±0.03 |
4.0199±0.0005 |
1.06±0.03 |
|
0.27238 |
1036.599 |
1552.44 |
305.00±0.03 |
4.0028±0.0005 |
1.15±0.03 |
|
0.31669 |
1042.225 |
1555.38 |
305.30±0.03 |
3.9661±0.0005 |
1.44±0.03 |
|
|
Cloxa+water+NaCl(0.20058m) |
||||
|
0.00000 |
1002.042 |
1531.63 |
– |
4.2541±0.0006 |
– |
|
0.01466 |
1004.329 |
1533.79 |
300.90±0.40 |
4.2325±0.0006 |
-2.00±0.60 |
|
0.07899 |
1013.985 |
1541.70 |
302.74±0.10 |
4.1492±0.0005 |
-0.70±0.10 |
|
0.11657 |
1019.421 |
1545.22 |
303.20±0.06 |
4.1083±0.0005 |
-0.03±0.07 |
|
0.15175 |
1024.219 |
1548.05 |
304.64±0.05 |
4.0742±0.0005 |
0.58±0.05 |
|
0.19914 |
1030.754 |
1551.77 |
304.61±0.04 |
4.0289±0.0005 |
0.99±0.04 |
|
0.23176 |
1035.045 |
1554.16 |
305.06±0.03 |
3.9999±0.0005 |
1.26±0.03 |
|
0.28156 |
1041.314 |
1557.65 |
306.02±0.03 |
3.9580±0.0005 |
1.62±0.03 |
|
|
Cloxa+water+NaCl(0.30012m) |
||||
|
0.01027 |
1005.835 |
1537.12 |
– |
4.2078±0.0005 |
– |
|
0.03577 |
1007.442 |
1538.76 |
300.00±0.80 |
4.1922±0.0005 |
-2.50±0.70 |
|
0.08030 |
1011.349 |
1542.23 |
301.20±0.00 |
0.0000±0.0000 |
-1.50±0.00 |
|
0.12389 |
1017.875 |
1547.00 |
303.40±0.10 |
4.1051±0.0005 |
-0.26±0.09 |
|
0.16407 |
1024.089 |
1550.85 |
304.06±0.07 |
4.0600±0.0005 |
0.48±0.06 |
|
0.19320 |
1029.646 |
1554.01 |
304.55±0.05 |
4.0217±0.0005 |
0.97±0.04 |
|
0.23903 |
1033.459 |
1556.09 |
305.49±0.04 |
3.9961±0.0005 |
1.31±0.04 |
|
0.26144 |
1039.426 |
1559.37 |
306.08±0.03 |
3.9565±0.0005 |
1.66±0.03 |
|
0.01027 |
1042.369 |
1561.01 |
305.97±0.03 |
3.9370±0.0005 |
1.75±0.03 |
|
|
Cloxa+water+NaCl(0.40623m) |
||||
|
0.00000 |
1009.984 |
1543.16 |
– |
4.1578±0.0005 |
– |
|
0.01265 |
1011.970 |
1545.25 |
298.80±0.60 |
4.1384±0.0005 |
-2.80±0.60 |
|
0.04111 |
1016.243 |
1549.00 |
302.20±0.20 |
4.1011±0.0005 |
-1.30±0.20 |
|
0.10324 |
1025.172 |
1554.15 |
304.54±0.07 |
4.0385±0.0005 |
0.86±0.07 |
|
0.11808 |
1027.298 |
1555.49 |
304.37±0.06 |
4.0232±0.0005 |
0.96±0.06 |
|
0.17294 |
1034.861 |
1560.17 |
304.82±0.04 |
3.9699±0.0005 |
1.34±0.04 |
|
0.18248 |
1036.192 |
1560.43 |
304.64±0.04 |
3.9634±0.0005 |
1.53±0.04 |
|
0.24694 |
1044.550 |
1564.40 |
305.65±0.03 |
3.9118±0.0005 |
2.09±0.03 |
|
0.29255 |
1050.206 |
1566.47 |
306.35±0.03 |
3.8804±0.0005 |
2.50±0.03 |
|
|
Cloxa+water+NaCl(0.50572m) |
||||
|
0.00000 |
1013.645 |
1548.47 |
– |
4.1144±0.0005 |
– |
|
0.02042 |
1016.819 |
1551.41 |
299.50±0.40 |
4.0860±0.0005 |
-1.50±0.30 |
|
0.03887 |
1019.579 |
1553.68 |
301.30±0.20 |
4.0631±0.0005 |
-0.80±0.20 |
|
0.04994 |
1021.205 |
1555.04 |
302.10±0.10 |
4.0495±0.0005 |
-0.60±0.10 |
|
0.07263 |
1024.417 |
1557.18 |
304.10±0.10 |
4.0257±0.0005 |
0.20±0.10 |
|
0.09956 |
1028.180 |
1559.41 |
305.24±0.07 |
3.9995±0.0005 |
0.82±0.07 |
|
0.13802 |
1033.397 |
1562.24 |
306.45±0.05 |
3.9649±0.0005 |
1.46±0.05 |
|
0.21541 |
1043.699 |
1567.77 |
306.81±0.03 |
3.8982±0.0005 |
2.06±0.03 |
|
0.25704 |
1049.040 |
1570.61 |
306.96±0.03 |
3.8643±0.0005 |
2.26±0.03 |
|
|
Cloxa+water+NaCl(0.60480m) |
||||
|
0.00000 |
1017.540 |
1554.11 |
– |
4.0690±0.0005 |
– |
|
0.03186 |
1022.407 |
1558.55 |
301.00±0.50 |
4.0266±0.0005 |
-1.00±0.20 |
|
0.06342 |
1026.985 |
1561.87 |
303.30±0.20 |
3.9916±0.0005 |
0.10±0.10 |
|
0.10141 |
1032.314 |
1564.92 |
304.84±0.10 |
3.9555±0.0005 |
1.06±0.07 |
|
0.14013 |
1037.516 |
1567.70 |
306.28±0.07 |
3.9217±0.0005 |
1.68±0.05 |
|
0.19528 |
1044.759 |
1571.46 |
307.14±0.05 |
3.8759±0.0005 |
2.19±0.04 |
|
0.23221 |
1049.570 |
1574.03 |
307.09±0.04 |
3.8456±0.0005 |
2.35±0.03 |
|
0.25649 |
1052.689 |
1575.68 |
307.01±0.03 |
3.8262±0.0005 |
2.44±0.03 |
|
0.29076 |
1056.876 |
1577.87 |
307.42±0.02 |
3.8004±0.0005 |
2.60±0.03 |
|
0.35519 |
1064.600 |
1582.11 |
307.77±0.02 |
3.7527±0.0005 |
2.80±0.02 |
Table 3: Fitting values or parameters of eq 5 for and with respect to NaCl molality at 298.15 K and Atmospheric Pressurea.
|
m/mol.kg-1 (NaCl) |
ϕv0 |
Sv1 |
Sv2 |
CMC ϕv |
ϕk0 |
Sk1 |
Sk2 |
CMC ϕk |
|
0.00000 |
302.82±0.03 |
8.52±0.24 |
5.08±0.32 |
0.212±0.012 |
-2.15±0.01 |
12.49±0.10 |
5.74±0.14 |
0.209±0.003 |
|
0.10049 |
302.57±0.02 |
10.24±0.21 |
6.64±0.24 |
0.173±0.008 |
-2.13±0.04 |
15.56±0.38 |
6.00±0.43 |
0.174±0.006 |
|
0.20058 |
300.57±0.39 |
25.00±4.80 |
11.80±3.70 |
0.147±0.040 |
-2.27±0.04 |
19.43±0.46 |
8.02±0.35 |
0.145±0.004 |
|
0.30012 |
299.48±0.25 |
48.60±4.90 |
15.40±2.20 |
0.082±0.011 |
-2.75±0.11 |
31.50±2.20 |
9.30±1.00 |
0.097±0.008 |
|
0.40623 |
297.29±0.42 |
119.00±14.00 |
10.00±1.70 |
0.054±0.005 |
-3.47±0.08 |
52.70±2.60 |
8.76±0.32 |
0.077±0.003 |
|
0.50572 |
297.78±0.42 |
87.30±8.50 |
9.60±2.60 |
0.089±0.006 |
-2.02±0.09 |
29.30±1.50 |
6.90±1.10 |
0.114±0.006 |
|
0.60480 |
299.46±0.40 |
54.70±5.60 |
6.10±1.70 |
0.127±0.009 |
-1.88±0.16 |
29.50±2.30 |
5.01±0.67 |
0.122±0.007 |
Conclusions
This work reports experimental information about the molecular interactions and aggregation behavior of cloxacillin sodium in water and in sodium chloride solution at 298.15K. From the measurement apparent molar volume, , apparent molar adiabatic compressibility, , limiting apparent molar volume, , limiting apparent molar adiabatic compressibility, , experimental slopes Sv , Sk and critical micelle concentration (CMC) of cloxacillin sodium were calculated and explain in terms of solute-solute, solute-solvent interactions and aggregation behavior. Thedecreases with the increase in NaCl concentration due to the disruption of hydrophobic hydration layer of cloxacillin sodium due to ion-hydrophobic interactions and increases for the ion-ion, ion-hydrophilic interactions. The shows same trend with respect to increasing NaCl concentration which supports. At first critical micelle concentration were found decreasing with respect to NaCl concentration due to minimization of electrostatic repulsion among the charged head groups, and increase in CMC at higher NaCl concentration is due to shape changes of micelles from spherical to different new, i.e cylindrical rod. So, apparent molar volume, limiting apparent molar volume and CMC gives us appreciated information about solute-solvent, solute-solute interaction and aggregation actions, which is very important for our body plasma co-solute interactions. Various ultrasound parameters support the existence of strong solute-solute, solute – solvent interactions and micelle phenomena. The results obtained from these studies can thus be helpful for pharmacological application of drugs.
Acknowledgments
The researchers show gratitude to Department of Chemistry, University of Rajshahi, Bangladesh for given that top laboratory accommodations to convey this research work.
Conflict of Interest
The researchers declare that they have no conflict of interest.
Reference
- Sarkar, A.; Pandit, B. K.; Sinha, B. J. Chem. Thermodyn.2016, 96, 161–168.
CrossRef - Devunuri, N.; Kancherla, S.; Chennuri, B. K.; Gardas, R. L. J. Mol. Liq.2016, 216, 347–353.
CrossRef - Scott, M. J.; Jones, M. N. Biochim. Biophys. Acta – Biomembr.2000, 1508 (1–2), 235–251.
CrossRef - Mincy, J. W.; Hem, S. L. Journal of Pharmaceutical Science.1975,65(8), 1165-1170.
CrossRef - Hartman, B.; Tomasz, A. Antimicrob. Agents Chemother.1981, 19 (5), 726–735.
CrossRef - Mays, D. L. Anal. Profiles Drug Subst. Excipients.1975, 4 (C), 113–136.
CrossRef - Lei, W.; Li, Z. J. Chem. Technol. Biotechnol.2004, 79 (3), 281–285.
CrossRef - Roy, M. N.; Dewan, R.; Roy, P. K.; Biswas, D. J. Chem. Eng. Data2010, 55 (9), 3617–3624.
CrossRef - Zhuo, K.; Zhang, Q.; Xuan, X.; Zhang, H.; Wang, J. Front. Chem. China2007, 2 (2), 193–198.
CrossRef - Chauhan, S.; Kumar, K.; Singh, K.; Jyoti, J. J. Surfactants Deterg.2013, 17 (1), 169–175.
CrossRef - Bahadur, I.; Deenadayalu, N. J. Solution Chem.2011, 40 (9), 1528–1543.
CrossRef - Khatun, M. R.; Islam, M. M.; Rima, F. R.; Islam, M. N. J. Chem. Eng. Data. 2016, 61 (1), 102–113.
CrossRef - Khatun, M. R.; Islam, M. M.; Islam, M. D. N.; Rhaman, M. D. M.; Nath, R. K. Asian J. Chem.2019, 31 (5), 1113–1127.
CrossRef - Chauhan, S.; Rana D. S.; Akash.; Rana K.; Chauhan, M. S.; Ahmad Umar. Advanced Science Letters. 2012, 5, 1-4.
CrossRef - Naseem, B.; Jamal, A.; Jamal, A. J. Mol. Liq.2013, 181, 68–76.
CrossRef - Elmolla, E. S.; Chaudhuri, M. Desalination.2010, 252 (1–3), 46–52.
CrossRef - Paula V. Messina.; Jose Miguel Besada-Porto.; Juan M. Ruso. Current Topics in Medicinal Chemistry. 2014, 14, 555-571.
CrossRef - Taboada, P.; Attwood, D.; Ruso, J. M.; Sarmiento, F.; Mosquera, V. Langmuir.1999, 15 (6), 2022–2028.
CrossRef - Talwar, G. P.; Srivastava, L. M. Textbook of Biochemistry and Human Biology; Prentice-Hall of India, 2003.
- Franks. Water Science Reviews. Hydration Phenomena in Colloidal Systems. 1989, 172.
CrossRef - Frank, H. S.; Wen, W.Y. Discuss. Faraday Soc.1957, 24, 133.
CrossRef - Masterton, W. L.; Volume, F.; Molal, P. J. Chem. Phys.1954, 22 (11), 1830.
CrossRef - Noriyuki, Y,;Kensuke L,; Susumu O. J. Chem. Phys.2006, 124, 184901.
- Alargova, R. G.; Danov, K. D.; Petkov, J. T.; Kralchevsky, P. A.; Broze, G. Langmuir,1997, 7463 (14), 5544–5551.
CrossRef - Sammalkorpi, M.; Karttunen, M.; Haataja, M. J. Phys. Chem.2009, 5863–5870.
CrossRef - Attwood, D. Adv. Colloid Interface Sci.1995, 55, 271–303.
CrossRef

This work is licensed under a Creative Commons Attribution 4.0 International License.









