Use of Hydroxyapatite from Eggshell as Raw Material for Synthesis Bone Graft
Sari Edi Cahyaningrum*, Nuniek Herdyastuti, Bella Devinaand Meita Kurniasari
Department of Chemistry, Faculty of Mathematics and Natural Science, Universitas Negeri Surabaya, 60231- Indonesia.
Corresponding Author E-mail: saricahyaningrum@unesa.ac.id
DOI : http://dx.doi.org/10.13005/ojc/350334
Article Received on : 21-10-2018
Article Accepted on : 10-05-2019
Article Published : 20 May 2019
In this research hydroxyapatite was synthezed from eggshell. The hydroxyapaptite was combined with Collagen and Chitosan for produce composite hydroxyapaptite/Coll/Chi by by ex-situ methods. The composite HA/Coll/ Chi was characterized such as a functional group and phase crystallinity and surface morphology. HA synthesized from the precursor calcium derived from eggshell reacted with H3PO4, HA sintered at 900°C. Result from spectra Infra red analysis showed that there are functional group PO43- and OH- that characteristic for HA. The bonding of HA with chitosan and collagen was showed from as well as a shift wave number in the group C = O and NH2. Results of XRD analysis showed that compositon of chitosan has effect in crystalinity of bone graft.
KEYWORDS:Bone Graft; Eggshell; Ex-Situ; Hydroxyapaptite
Download this article as:| Copy the following to cite this article: Cahyaningrum S. E, Herdyastuti N, Devina B, Kurniasari M. Use of Hydroxyapatite from Eggshell as Raw Material for Synthesis Bone Graft. Orient J Chem 2019;35(3). |
| Copy the following to cite this URL: Cahyaningrum S. E, Herdyastuti N, Devina B, Kurniasari M. Use of Hydroxyapatite from Eggshell as Raw Material for Synthesis Bone Graft. Orient J Chem 2019;35(3). Available from: https://bit.ly/30v98kx |
Introduction
Bone as part of human body has very important function, so that in the event of fracture bone will cause the problem of serious health and needed technique healing for correct bone. One of the techniques for healing of bone is use synthetic bone graft biomaterial. The good synthetic of bone graft have to biocompatible, bioactive, nontoxic, supporting the nature of and osteoconductive of osteoinductive,1-2 beside that the bone graft to have composition and structure which is equal with bone, that is containing > 69 % calcium phosphate, especially hidroxsiapatite; 21% collagen; 9% water and 1% other component.3 Hidroxyapatite as especial component have the power of unfavorable mechanic, brittle and breakable, so that in synthesis of synthetic bone graft, the hydroxyapatite has to be composite with other material. Composite of polymer matrix have some advantage among others avoid the problem of shielding stress and eliminate second surgery procedure to eliminate implant. Mineral phase especially function of hidroxyapatite give inertia and delaying, while organic matrix give interest strength and bone flexibility.8 Collagen measure up to good biocompatibility to degradation and permeated by body. In composite material, collagen and hidroxyapatite play role which is same as at natural bone.4 The collagen can isolation from various sources of that are ox bone, goat, scrawl and fishbone. At this research the collagen got from scrawl. Characteristics osteoconductive and osteointegrated of graft bone relate to porosity storey level and porosity size. To form pore at graft bone need materials that added which is function as porogen. Porogen that use must have characteristic biocompatible, biodegradable and nontoxic, one of them is chitosan.
Chitosan is one of the natural polymers that is produce from chitin by deacetilazing processes. Chitosan has potency for filler in composite making. Chitosan can function as porogen. It’s has characteristic non toxic, biocompatible and can improve osteoconductive bone.5-7 In this research the bone graft was made by composite three materials that is hidroxyapatite/collagen of chitosan with mass composition variation of which look like with composition a period of bone. The syntheses there of bone graft are two methods able to be used are ex-situ and in-situ.8 Method of ex-situ represent method addition of polymer when collagen, chitosan after especial materials in the form of hidroxyapatite have been formed, while at method of in-situ addition collagen and chitosan done at the time of process of synthesis hidroxyapatite take place. Some research indicates that method of ex situ yield product having compared to higher purity than other method.
Experimental
The calcium oxide was isolated from eggshell (Gallus gallus), the collagen was synthesis from scrawl (Gallus gallus) obtained from Mojosari, Indonesia. Chitosan (85% DD), Acetic acid, nitrate acid, natrium hydroxide p.a quality purchase from Merck, Hydroxyapaptite standart (HAp-Std) from Bank Jaringan Dr. Soetomo Hospital, Surabaya, Indonesia.
Synthesis of Hydroxyapatite
Synthesis hydroxyapatite was done by reacting calcium precursor and precursor phosphate with comparison of concentration of Ca/P 1.67. CaO powder was added by aqudemineral to yield hydroxide calcium. After that it was added with phosphate and stirrer until homogeneous. And then the solution added with natrium hydroxide until pH 10. Mixture was aging at room temperature during 24 hours. The solution yielded to filter and its sediment is washed with aquademineral, dried at oven in temperature 110°C during 2 hours. After that, it was added with nitrate acid and sintering in 900°C during 2 hours. The crystal that produces was cooled in furnace and produce hydroxyapatite.
Synthesis of Hydroxyapatite- Collagen- Chitosan Bone Graft
Amount of chitosan was dissolved in acetate acid solution. It solution was stirred until homogeneous. Hydroxyapatite powder have been dissolved with water was added in chitosan solution with wish drop method. After that it solution was added with collagen solution. The composite that produce was treatment with freeze dry and then condensed on natrium hydroxide solution. After that the composite was dried in freeze dry again. The processed repeat again for composite with variation Massa composition HA/Coll/Chi 7:1.5:1.5 and 7:1:2. Composite HA/Coll/Chi was characterized functional group and crystalinity.
Results and Discussion
At hidroxyapatite synthesis from an eggshell, the eggshell must be calcinazed to change CaCO3 became CaO. CaO powder was soluted in aquademineral form Ca(OH)2 solution. It solution was added with phosphoric acid solution drop to drop so that pH not go down drastically. The rate of phosphoric acid addition very related to obtained pH in the end of synthesis. Degradation at pH under 7 causing imperfect dissociation of phosphoric acid so that yield β-Ca3(PO4)2 and CaO. Phosphoric acid solution that was added with slowly can increase the homogeneity of solution.9,10 At the research, the synthesis of hydroxyapatite is undergone at temperature 60°C, this function is to crystal maximalized produce and reduces produce monoclinic crystal structure. The monoclinic crystal structure can produce in temperature under at 60°C.11 Hydroxyapatite that synthesis is expected has structure same with bone structure, there are hexagonal structure. When phosphoric acid was added in calcium hydroxide solution the solution slowly condensation become to have the character of acid, while process of crystallization take place effective at base condition12 that the solution must be added with natrium hydroxide until pH 10, hydroxyapaptite crystal stabile at pH 10.13
The hydroxyapatite from synthesis with variation sintering temperature compared with hydroxyapatite standard from Bank Jaringan. Fig 2 showed the spectra of CaO, hydroxyapatite that sintering at 800 (Hydroxyapaptite-800); hydroxyapatite-900; hydroxyapaptite-1000°C; hydroxyapatite without sintering and hydroxyapatite standart from Bank Jaringan (HAp-std).
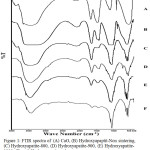 |
Figure 1: FTIR spectra of (A) CaO, (B) Hydroxyapaptit-Non sintering, (C) Hydroxyapatite-800, (D) Hydroxyapatite-900, (E) Hydroxyapatite-1000, (F) and Hydroxyapatite-std. |
Sample HAp-TS has characteristic spectra at wave number 411.07; 1095; 576.97; 1400.36; 659.96; and 1648.76 cm-1. At Fig.2B spectra don’t band at 630 cm-1 because these phase HAp has more impurities than HAp that was undergo sintering processes. The characteristic of HAp-800 are have peak at 963.26; 495.2; 1070.5; 570; 602.5; 1420.21; 631.27; 1637.49; and 3401.04 cm-1. Spectra Fig.1D. has band at around 454.76; 1072.5; 528.5; and 163801 cm-1. The characteristic spectra for Fig.1E there are have spectra at around 403.26; 1074.5; 575.5; 1638.01 and 3435,21 cm-1. The wave number for spectra samples A-F are similar there is characteristic for CO32-, PO43-, and OH– functional groups.
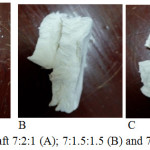 |
Figure 2: Bone graft 7:2:1 (A); 7:1.5:1.5 (B) and 7:1:2 (C). |
At Figure 2 seen that all bone graft have physical character of solid with white in colour. All bone graft has hollow fiber which result bone graft do not too hard when depressed. Pursuant to perception of physical seen bone graft have been mingled is homogeneously. Bone graft A with content of chitosan at most brass white in color, more and more content of chitosan hence its color tend to turn white brass. Bone graft C also more solid and resilient when compared with bone graft B and bone graft A. Progressively the increasing of chitosan the bone graft seen it more resilient.
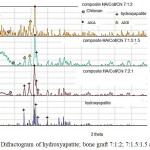 |
Figure 3: Difractogram of hydroxyapatite; bone graft 7:1:2; 7:1.5:1.5 and 7:2:1. |
At Figure 3 saw the difractogram of hydroxyapaptie was dominant hydroxyapatite phase, with highest difractograme at 31.76o and 31.8o. This phase there are in three bone graft. Beside phase hydroxyapatite in this research produse another phase there are apatite carbonat type A and apatite carbonat type B. The highest intensity of difractogram is 100% for bone graft HA/Coll/Chi 7:2:1 with 2θ eangle is = 32.14o and 32.08o. The bone graft HA/Coll/Chi 7:1.5:1.5 and HA/Coll/Chi 7:1:2, the phase dominant is apatite carbonat type A with 2θ = 32.32o and 32.4o. The Hydroxyapatite phase on bone graft HA/Coll/Chi 7:1.5:1.5 is relatife medium there at 85.57 % and 2θ = 32.18o, while on bone graft HA/Coll/Chi 7:1:2 phase of hydroxyapaptite at 2θ = 32.18o.
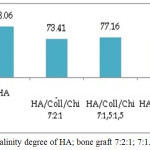 |
Figure 4: Crystalinity degree of HA; bone graft 7:2:1; 7:1.5:1.5 and 7:1:2. |
At Figure 4 showed that material organic has effect in crystalinity, addition of collagen influence degree of crystalinity. Graft Bone with amount of at most of collagen, the crystalinity is lowest. Collagen is organic material with amorf character.
Conclusion
Hydroxyapatite that produce from eggshell with precipitation wet method have functional group similar with hydrxyapatite standart from Bank Jaringan, there are characteristic for CO32-, PO43-, and OH– functional groups. The hydroxyapatite was composed with collagen and chitosan to produce bone graft Ha/Coll/Chi. Graft Bone with amount of at most of collagen, the crystalinity is lowest.
Acknowledgements
We would like to thank to DRPM DIKTI which has provided financial support through National Priority Research Programme, Hibah Penelitian Berbasis Kompetensi Programme 2018 with No. Contract: 000001.92/UN38.11-P/LT/2018, February 12, 2018
References
- Jamarun, N; Azharman, Z; Arief, S; Sari, T.P; Asril, A; and Elfina, S; Rasayan Journal of Chemistry. 2015, 8, 133-137
- Oshida; Yoshiki; Wang, Sun, C; Qu and Liang, K; Hydroxyapapite Synthesis and Apllication. New York: 2015. Momentum Press.
- Cahyaningrum, S.E; Herdyatuty, N; Supangat, D; Devina, B; and Kurniasari, M. Rasayan Journal of Chemistry. 2018, 11, 488-493
- Pallela, R; Sreedhar, B; and Janapala, VR.1 Int J Biol Macromol. 2011, 49, 85-92.
- Halabalova, V; Simek, L; and Mokrejs, P. Rasayan Journal of Chemistry, 2011, 4, 223-241.
- Huang, Z; Feng, Q; Yu, B; and Li S.Materials Science and Engineering C. 2011, 31, 683–687.
- Puntharoda, R; Sankrama, C; Chantarameec, N; Pookmaneea,P; and Haller, K.J. Journal of Ceramic Processing Research. 2013, 14, 198-201.
- Wattanutchariya, W and W. Changkowcha, W. 2014. Proceedings of the International MultiConference of Engineers and Computer Scientists. IMECS Hong Kong, 2014, 2, 12 – 14, 2014.
- Gou, Q; Reza, G; Thomas, W; Andreas, A; Evgeny, L; Cemal, E; Olaf, M; Wei, C; Boris,C; and Andreas; O. Polymers, 2014.6. 2037-2055.
- Tangboriboon, N; Khongnakhon,T; Kittikul,S; Kunanuruksapong, R and Sirivat, A. J. Sol-Gel Sci. Technol. 2011.58. 33-41.
- Anita L.J;, Ravichandran, K and Sundareswari, M. Journal of Chemical and Pharmaceutical Research, 2015.7 .231-239.
- Malina, D; Biernat, K and Kupiec, A.S. Acta Bichimica Polonica. 2010.60 851-855.
- S.V Dorozhkin, Bioceramics of Calcium Orthophosphates. Biomaterials, 2010, 31, 1465-85.

This work is licensed under a Creative Commons Attribution 4.0 International License.









