Preparation of Coal Briquettes and Determination of Their Physical and Chemical Properties
M. I. Tulepov1,2, L. R. Sassykova*1,2  , A. R. Kerimkulova1,2, G. O. Tureshova1,2, D. M. Tolep1,2, A. O. Zhapekova1, G. A. Spanova1, F. Yu. Abdrakova1,2 and Z. A. Mansurov1,2
, A. R. Kerimkulova1,2, G. O. Tureshova1,2, D. M. Tolep1,2, A. O. Zhapekova1, G. A. Spanova1, F. Yu. Abdrakova1,2 and Z. A. Mansurov1,2
1Al-Farabi Kazakh National University, 71, al-Farabi ave., 050040, Almaty, Kazakhstan.
2The Combustion Problems Institute, 172, Bogenbaibatyr str., 050012, Almaty, Kazakhstan.
Corresponding Author E-mail: larissa.rav@mail.ru
DOI : http://dx.doi.org/10.13005/ojc/350120
Article Received on : 11-11-2018
Article Accepted on : 08-12-2018
Article Published : 09 Feb 2019
The purpose of this study was to prepare briquettes based on coal from the Oy-Karagay deposit (Kazakhstan) and polymers, and to determine their physicochemical and technological properties. Coal briquettes were prepared according to the classical technology, which consists of grinding coal, drying it to a certain moisture, mixing with binders, heating the coal charge, pressing and cooling. It was found that mechanical dispersion of coal briquette with PET does not cause the destruction of aggregates. After the briquette calcination the main phase remains the amorphous carbon phase with the mineral component in the form of iron oxide, silicon dioxide, calcite, feldspar and Fe2SiO4. As a result of the tests, a technology for the production of coal briquettes was developed with the choice of the technological mode and the optimal composition of components.
KEYWORDS:Brown Coal; Binding Materials; Coal Briquettes; Oy-Karagay Field; Polyethylene Terephthalate
Download this article as:| Copy the following to cite this article: Tulepov M. I, Sassykova L. R, Kerimkulova A. R, Tureshova G. O, Tolep D. M, Zhapekova A. O, Spanova G. A, Abdrakova F. Y, Mansurov Z. A. Preparation of Coal Briquettes and Determination of Their Physical and Chemical Properties. Orient J Chem 2019;35(1). |
| Copy the following to cite this URL: Tulepov M. I, Sassykova L. R, Kerimkulova A. R, Tureshova G. O, Tolep D. M, Zhapekova A. O, Spanova G. A, Abdrakova F. Y, Mansurov Z. A. Preparation of Coal Briquettes and Determination of Their Physical and Chemical Properties. Orient J Chem 2019;35(1). Available from: https://bit.ly/2Gvoz4M |
Introduction
In Kazakhstan, brown coal reserves amount to 12.1 billion tons: deposits of Karaganda, Sarykol, Turgai, Kolzhatsky, Nizhneiliysky, Shubarkulsky, Lengersky, Maykubensky, Oy-Karagay, Kiyaktiyansky and Karazhyra basins.1-3 As a rule, the content of fines in the coal produced in some cases is up to 70%, and, as a rule, it is not used as fuel. A significant amount of coal fines is lost and during transportation – is carried away by the wind, drops out of the vehicle. Coal fines accumulate in large quantities at coal mining sites, at transshipment points and in most cases occupy useful areas, creating the threat of pollution of the surrounding area. The feasibility of briquetting coal fine is due to its fine condition and the complexity of transportation, the impossibility of burning in standard grate furnaces. The efficiency of heat briquette use is 75% compared to 46.7% for raw coal containing fines.4-6 The main task in the production of coal briquettes is to make the most complete mixing of coal fines with tar, which for economic reasons must be taken in a minimum quantity, but, however, in such that a compact mass is obtained when pressing briquettes. The amount of resin needed to form briquettes depends both on the properties of the resin itself and on the properties of the coal taken. To reduce the amount of harmful emissions during coal briquetting, various additives can be added, for example, camphor, naphthalene or nitrobenzene to eliminate the formation of smoke during combustion. During the disintegration of the molecules of the binder, unsaturated compounds are formed, which easily react with the aromatic constituent of coal. And as a result, heavier molecules that are not capable of volatilization are formed, which decompose again with further heating and again smoke generation is significantly reduced due to it.7
The purpose of this study was to prepare briquettes based on coal from the Oy-Karagay deposit (Kazakhstan) and polymers, and to determine their physicochemical and technological properties.
Materials and Methods
Coals of the Oy-Karagay deposit of Kazakhstan were chosen as the object of research for the production of briquettes from coal and polymers. Coal briquettes were prepared according to the classical technology, which consists of grinding coal, drying it to a certain moisture, mixing with binders, heating the coal charge, pressing and cooling. For experiments, mixtures of solid polymer residues were preliminarily heat treated. Then, together with coal, mechanical treatment was carried out to a particle size of > 200 μm. The obtained mixtures were carefully stored with the observance of precautionary measures in order to protect from oxidation, possible sources of contamination, which could be reagents, the atmosphere, dust. Toluene, benzene, alcohol benzene, naphthalene, acetone were used as solvents. To ensure the plasticity of the charge and liquefaction of the binder before briquetting, a fixed volume of water was added to it. The mixture prepared for pressing was tested for moisture content.
The IR absorption spectra of the samples under study were measured on a PerkinElmer Spectrum 65 IR spectrometer in the region of 700-3,600 cm-1, as well as in a UV – 750U spectrophotometer in the region of 400-4,000 cm-1. X-ray fluorescence studies and elemental analysis of a solid sample were performed on a FOCUS-М2М microanalyzer using Fe radiation in the range from 2 to 37 V. The intensity of the diffraction peaks was estimated by an analytical method in a tetragonal syngony. Changes in the surface and structure were detected using an Ntegra Therma.
A hydraulic automated press IP-100 was used for briquetting. Pressing was carried out in closed cylindrical dies with a diameter of 25 mm. Heat treatment of briquettes was carried out in SNOL-3.5 cabinets with adjustable temperature in the range of 50-400°С. The estimated number of components of briquetting in a certain sequence was mixed to obtain a homogeneous mass and was briquetted in a press at a pressing pressure of 25 MPa, which corresponds to the pressure developed in industrial roller presses.
The strength of the briquettes was estimated by the crushing method on the IP-100 press with control of the maximum effort that the briquette withstands. Determination of the strength characteristics was carried out after cooling the briquettes to room temperature.
Results and Discussion
Technological Characteristics of the Components of the Coal Briquette
The energy characteristics of the original coal from the Oy-Karagay field were determined: calorific value – 6,700 kcal / kg; high calorific value – 3,600 kcal / hour; the yield of volatile – 27 – 45%; analytical humidity – 7.8%.
Coals of the Oy-Karagay field are characterized by average technical strength. Commercial coal consists on average of 56-64% of coal of size 13mm and 30% of class less than 6 mm. It should be noted that the coals during long-term storage and storage dry up and turn into coal fines, unsuitable for consumption. This phenomenon involves the use of coal fines briquetting technology.8-10 Low ash and high calorific value (tab.) are also factors that positively affect the quality characteristics of the coal briquette.
Table 1: Technological characteristics of coal from the Oi-Karagay field.
| Wa, % | Va, % | Aa, % | Stotal, % | N, % | C, % | O, % | H, % | , kcal / hour | q, kcal / kg |
| 7.8 | 27-45 | 12.5 | 0.6-1.1 | 0.7-1.2 | 75- 83 | 15-37 | 3.0-6.5 | 3,600 | 6,700 |
Note: Wa, Va, Aa are moisture, volatile matter and ash content in an analytical state; Stotal, N, C, O, H are contents of sulfur, nitrogen, carbon and hydrogen; is a high calorific value; q is calorie.
Search of new, more economic and high-quality binding materials is one of the major problems. In the process of researching the production of briquettes from the Oy-Karagay coal, it was decided to consider the possibility of using synthetic waste – polyethylene terephthalate (PET) as a binder. Use of synthetic waste as coal, binding at briquetting, allows to increase heat of combustion of coal and at the same time to involve synthetic waste which occupy the huge spaces and polluting the environment in processing. As well as the use of polyethylene terephthalate gives briquettes plasticity, which causes high strength. This quality is very convenient when transporting briquettes. This polymer simultaneously plays the role of fuel, which contributes to better burning.
To prepare the mixture, coal with a moisture content of 3.0% was crushed in the mill and dispersed on the screens.
The Study of the Physico-Chemical Properties of Coal Briquettes
The SEM data show (fig. 1, 2) that in the initial angle after calcination, a decrease in the size of its particles and aggregation of particles with the formation of large aggregates with a size of 227.3- 289.9 nm occur simultaneously.
 |
Figure 1: Initial coal of the field Oy-Karagay. |
 |
Figure 2: Briquette after calcination. |
PET waste is a semi-solid viscous mass that retains the state of aggregation at a temperature of 25-30°С. Consequently, when using them as a binder and obtaining a mixture of a homogeneous composition, heating to the melting point of 120-130°C will be required. The data of SEM of coal with PET showed (fig.3) that these compounds belong to amorphous formations. In particular, the organic component of the coal fines is a mixture of various X-ray amorphous components, the presence and quantity of which varies in a series of metamorphism.
Mechanical dispersion of coal briquette with PET does not cause the destruction of aggregates (fig.3). The XRD data of the briquette after calcination11-13 showed that the main phase remains the amorphous carbon phase with the mineral component in the form of iron oxide, silicon dioxide, calcite, feldspar and Fe2 SiO4. Phase analysis results were obtained under shooting conditions Fe Kα 28 KV, 28 MA. Samples of briquettes are practically X-ray amorphous, due to the carbon-containing phase, weak peaks from SiO2, Сo3O4 were also observed. Peaks 2.70, 2.50 Å refer to hematite Fe2O3 (ASTM 33-664); peaks 3.87; 3.07; 2.34, 2.27 Å refer to K2O2 (ASTM 32-827). Peaks: 4.26; 3.34; 2.45; 2.28; 2.23; 2.12; 1.81; 1.54 Å refer to α-quartz SiO2 (ASTM 5-490). Peaks: 3.80; 3.01; 2.27; 1,191; 1.87 Å refer to calcite CaCO3 (ASTM 5-586).
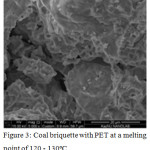 |
Figure 3: Coal briquette with PET at a melting point of 120 – 130ºС. |
The elemental composition of the initial coal was also investigated on the basis of technical analyzes and technological tests for waste of mineral raw materials with experimental burning of coal. Content, %: sulfur – 0.6-1.1%; carbon 75 – 83%; oxygen – 15 – 37%; hydrogen – 3.0 – 6.5%; nitrogen – 0.7 – 1.2%; humic acids – up to 80%.
IR spectroscopic data of the initial coal and coal together with PET (fig. 4) showed that after heat treatment of the coals, the number of saturated CH-bonds in the form of methylene and methyl groups increased in the range of 2,500 to 3,500 cm-1, one can see a noticeable decrease in the intensity of absorption in the region of 1,500 -1,600 cm-1. This results from the fact that in the course of heat treatment there are processes of decomposition of aromatic structure of coals which interact with structure of polymer. These changes12 are characterized by decrease in intensity of absorption in the field of 1,500-1,600 cm-1, and the appearance of new peaks in the field of 800-1,400 cm of cm-1. There is a destruction of the carboxyl groups, ketones and esters that make up the polymer and a decrease in the number of methylene structures.
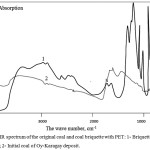 |
Figure 4: IR spectrum of the original coal and coal briquette with PET: 1- Briquettes of coal with PET; 2- Initial coal of Oy-Karagay deposit. |
The IR spectra data in the case of a briquette with a binder in the form of thermally caloric polyethylene terephthalate show that in the reaction products the intensity of the absorption bands of aliphatic hydrogen at 3,430 cm-1 and methyl groups at 1,397–1,585 cm-1 increases. It seems that the growth of hydroxyl groups is due to the breakdown of ester bonds, the increase in methyl groups is due to the addition of ethylene from polyethylene, and the growth of carbonyls is probably the result of acylation of aromatic fragments of coal. In the IR spectra12 the intensity of the absorption bands of the CH-aromatic groups increased (in the region of 467–540 cm-1); absorption decreased due to C-H stretching vibrations (methylene and methyl groups) in the 997 cm-1 range. The intensity of absorption corresponding to carbonyl groups in aldehydes, lactones, esters (1,611 cm-1) changed.
Considering the results of physical and chemical studies, we can assume the following stages of the process of briquetting coals with binders: at first, adsorption of the binder by the briquetted material and the formation of a thin film of the binder on the surface of the particles. Next, there are pressing processes, during which, under the influence of pressure, processes occur at the interface of the connecting agent with the surface of coal, the formation of structuring polycyclic complexes or layers around the granular carbon-containing component, determining the strength and other technical characteristics of fuel briquettes.
The last stage of the formation of the structure of briquettes is their hardening, accompanied by the processes of water dehydration and drying.
Studies of the physical parameters of briquettes and the data of a scanning electron microscope showed that the thickness of the adsorption layer of coal and the cohesion of the binder in thin layers play an important role in the formation of the structure and strength of the briquette.14-16
According to the data of SEM, it can be assumed that with the optimum thickness of the film layer, the maximum manifestation of capillary forces and an increase in the adhesive interaction between the particles and the binder take place.
In this work, the change in the compressive strength of the fuel briquette with varying moisture content of the charge and pressing pressure is also investigated.
It was established that with decreasing particle size, their bond strength in a briquette increases. With an excess of moisture in the coal, sticking of the binder to the surface of the particles becomes difficult and the strength of the briquettes decreases. With a very dry coal, the wettability of the surface of the particles by the binder deteriorates and its consumption increases. The optimum moisture content corresponding to the lowest binder consumption is determined empirically.
The Establishment of Optimal Parameters for the Briquetting of Coal Fines
Test of coal briquettes moisture resistance were carried out. The composition of the coal briquette – coal, cellulose and a binder (PET). PET content varied from 5 to 25%, cellulose content was constant – 15%. According to the test results, it was found that the optimum PET content in the coal briquette is 15-19%, and a further increase in the proportion of the binder is not advisable due to environmental and economic considerations. Fig. 5 shows the appearance of the coal briquette.
 |
Figure 5: Appearance of coal briquettes. |
The nature of the influence of humidity of the briquetted charge on the strength characteristics of fuel briquettes is established. In the test carried out, the pressing pressure was 160 MPa.
In the fig.6 the dependence of durability of a briquette on compression from humidity of furnace charge is presented. By the method of differentiation function on extremum points has investigated. The optimum value of humidity, equal 4.9% is established.
In the study of the dependence of the compressive strength of briquettes on the extrusion pressing pressure for a 15% binder content, a maximum was found at the point corresponding to the pressing pressure P = 156 MPa. When rounding, we obtain the optimal value of the pressing pressure, 160 MPa. A further increase in pressure leads to a material being repressed, and with a lower pressure the required strength is not ensured.17-20 With a 10% binder content, the strength of the briquette does not reach the standard value (7.5 MPa9), the optimum corresponds to P = 143 MPa.
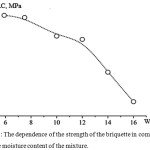 |
Figure 6: The dependence of the strength of the briquette in compression from the moisture content of the mixture. |
Conclusion
On the basis of the of Oy-Karagay deposit (Kazakhstan) were prepared coal briquettes and their technological characteristics and physicochemical properties had been studied. The nature of the influence of humidity of the briquetted charge on the strength characteristics of fuel briquettes and the optimum value of humidity equal to 4.9% are established.
It can be possible to assume the following stages of the process of briquetting coals with binders:
Adsorption of the binder by the briquetted material and the formation of a thin film of the binder on the surface of the particles,
Pressing processes, during which, under the influence of pressure, processes occur at the interface of the connecting agent with the surface of coal,
The formation of structuring polycyclic complexes or layers around the granular carbon-containing component, determining the strength and other technical characteristics of fuel briquettes.
Formation of the structure of briquettes is their hardening, accompanied by the processes of water dehydration and drying.
Based on the technological characteristics of coal from Oy-Karagay deposit, a technology has been developed for producing coal briquettes with the selection of the technological regime and the optimal component composition providing the necessary strength and low moisture absorption of the briquettes. The dependences of the compressive strength of coal briquettes on the briquetting parameters of the charge are obtained, which makes it possible to control the briquetting process and obtain a product with specified technological characteristics.
Acknowledgements
We would like to express sincere gratitude to the Ministry of Science and Education of the Republic of Kazakhstan for grant financing (Project 2018-2020).
Conflict of Interest
There is no conflict of interest.
References
- Kalechits, I.V., Solid Fuel Chemistry, 2001, 3, 3.
- Lenev, L.A.; Kuskov, V.B., Notes of the Mining Institute, 2006, 169, 147-149.
- Sassykova, L.R., Chemistry and physics of petroleum, gas and coal, Qazaq University, Almaty, 2017, 11, 66.
- Baiseitov, D.A.; Tulepov, M.I.; Tursynbek, S.; Sassykova, L.R.; Nazhipkyzy, M.; Gabdrashova, Sh. E.; Kazakov, Y. V.; Pustovalov, I. O.; Abdrakova, F.Y.; Mansurov, Z. A.; Dalton, A. B., Rasayan J. Chem., 2017, 10(2), 344-348.
- Antal, M.J.; Grønli, M., Industrial & Engineering Chemistry Research, 2003, 8, 1619-1640.
CrossRef - Perlack, R.D.; Stevenson, G.G.; Shelton, R.B. Prospects for coal briquettes as a substitute fuel for wood and charcoal in US Agency for International Development Assisted countries, United States: N. p., 1986.
CrossRef - Maloletnev, A.S.; Gyul’maliev, A.M., Solid Fuel Chemistry, 2013, 47(4), 231.
CrossRef - Somerville, M.A., Journal of Sustainable Metallurgy, 2016, 3, 228–238.
CrossRef - Shirshikov, V.I.; Piyalkin,V.N.; Litvinov, V.V., Chemistry and technology of wood-coal briquettes production, Khimizdat, SPb., 2012, 111.
- Khodakov, G. S.; Russian Chemical Reviews, 1963, 32(7), 386.
CrossRef - Baiseitov, D.A.; Gabdrashova, Sh.E.; Akylbai, A.K.; Dalelkhanuly, O.; Kudyarova, Zh.B.; Sassykova, L.R.; Tulepov, M.I.; Mansurov, Z.A., Int. J. Chem. Sci., 2016, 14(1), 261-268.
- Baiseitov, D.A.; Tulepov, M.I.; Sassykova, L.R.; Gabdrashova, Sh.E.; Magazova, A.N.; Dalelkhanuly, O.; Kudyarova, Zh.B.; Mansurov, Z.A. Bulgarian Chemical Communications, 2017, 49(3), 600-607.
- D.A. Baiseitov, Sh.E. Gabdrashova, A.N. Magazova, O.Dalelkhanuly, Zh.B.Kudyarova, M.I. Tulepov, L.R. Sassykova, Z.A. Mansurov, Int. J. Chem. Sci., 2016, 14(1), 244-250.
- Kudaibergenov, K.; Ongarbayev, Y.; Tulepov, M.; Mansurov, Z.; Ualiyev, Zh.; Kabdoldina, A.; Tuleshov, Y. Advanced Materials Research, 2014, 893, 478-481.
CrossRef - Jandosov, J.; Mansurov, Z.A.; Bijsenbayev, M.A.; Tulepov, M.I.; Ismagilov, Z.R.; Shikina, N.V.; Ismagilov, I.Z.; Andrievskaya, I.P. Advanced Materials Research, 2013, 602-604, 85-89.
CrossRef - Kathirvelu, B.; Subramanian, S.; Govindan, N.; Santhanam, S., Sustainable Environment Research, 2017, 27(6), 283-290.
CrossRef - Brooks, K.S.; Glasser, D., Fuel, 1986, 65(8), 1035-1037.
CrossRef - Shen, J. Coal conversion, 1999, 22(14), 5-8.
- Lecourtiev, J.; Lee, J. T., Colloids and surface, 1990, 47(3), 219–231.
CrossRef - Kim, Jae–Jo; Weler, S., Fuel Process Technol, 1985, 11(2), 205–209.
CrossRef

This work is licensed under a Creative Commons Attribution 4.0 International License.









