The Preparation of Nano-oxides of Zinc and Barium of [Ba (o-phen)4] [Zn (EDTA)], [Zn(o-phen)4]2 [Ba(SCN)6], and Synthesis of ZrO2 Nanoparticlesby Chemical Method
Mohammad Hakimi1, Hamideh Saravani2 and Abdoulhamid Arbabi1
1Department of Chemistry, Payame Noor University, 19395-4697 Tehran, I. R. Iran.
2Department of Chemistry, Sistan and Baluchestan University, P.O. Box 98135-674, University Street, Zahedan, I. R. Iran.
Corresponding Author E-mail: arbabihamid20@yahoo.com
DOI : http://dx.doi.org/10.13005/ojc/340351
Article Received on : February 22, 2018
Article Accepted on : March 28, 2018
Article Published : 31 May 2018
For the preparation of Nano-oxides of Zinc and Barium, first, the two-component complexes [Ba (o-phen) 4] [Zn (EDTA)], [Zn(o-phen)4]2 [Ba(SCN)6] were produced, then they were heated for one hour in 700 and 800°C inside the electrical furnacet. The complexes have been characterized by elemental analysis, FT-IR .To Synthesize nano-metric ZrO2, the chemical method was used, which was the most economical method. The minimum temperature for performing alkaline fusion using NaOH and KOH catalysts – with the ratio of twice the weight of Zirconium silicate as the optimum flux – was determined to be 850°C. Under optimum conditions, Zirconia powders were synthesized in 900°C with longetitivty of two hours.Using X-ray diffraction (XRD) and scanning electron microscopy (SEM), the Nanometric state of particles of Zirconium oxide, Barium ,and Zinc oxide was confirmed.
KEYWORDS:Barium Nano-oxides and Zinc; [Ba (o-phen) 4] [Zn (EDTA)]; [Zn(o-phen)4]2 [Ba(SCN)6]; Zirconia Powders; XRD; SEM
Download this article as:| Copy the following to cite this article: Hakimi M, Saravani H, Arbabi A. The Preparation of Nano-oxides of Zinc and Barium of [Ba (o-phen)4] [Zn (EDTA)], [Zn(o-phen)4]2 [Ba(SCN)6], and Synthesis of ZrO2 Nanoparticlesby Chemical Method. Orient J Chem 2018;34(3). |
| Copy the following to cite this URL: Hakimi M, Saravani H, Arbabi A. The Preparation of Nano-oxides of Zinc and Barium of [Ba (o-phen)4] [Zn (EDTA)], [Zn(o-phen)4]2 [Ba(SCN)6], and Synthesis of ZrO2 Nanoparticlesby Chemical Method. Orient J Chem 2018;34(3). Available from: http://www.orientjchem.org/?p=46308 |
Introduction
In recent years, significant advances have been made in the field of nanotechnology. By changing the composition and structure of nanoscale materials, a major highway has been developed for the production of new nano-materials with special properties.1 Nanometric powders are currently being studied, as their properties and applications are among the most recent research in the world.2 The synthesis of nano-ceramic powders is one of the most important areas in material production technology. Nanotechnology has led to the emergence of new achievements in medical science and engineering. Chemists, engineers, and physicians use this science at the molecular level to produce important advances in chemistry, biological sciences, healthcare and treatment.3 Nanotechnology applications are carried out in various fields, including the electronics industry, the construction of chips at very small sizes,4,5 the integration of nano and micro-mechanical tools for controlling defense systems,6 designing and manufacturing light weight, high-strength, and durable materials for airplanes, rockets and space stations,6,7 the development of green technologies for the removal of small contaminants from water and water resources, the improvement of biocompatible plantations and the process of drug discovery,8 the production of inorganic and inorganic synthetic nano-materials for the creation of a diagnostic function inside cells,9 increasing the efficiency of chemical reactions and reducing waste.10,11 ZnO nanoparticles, zirconium oxide, and barium oxide are commonly used minerals. Because of their physical and chemical properties such as high chemical stability, low dielectric constant, and high catalytic activity, are of high interest to artisans, and due to light, electronic, semiconducting properties in nanoscale and strong luminescence, they are also used in solar cells, diodes and sensors. The most important features of ZrO2 nanoparticles are fragility-resistant with the uniformity of particle dimensions and zirconia being an electrical and thermal insulator. Since the thermal expansion coefficient of this material is similar to steel, it can be used instead of steel. Yet unlike steel, it is resistant to corrosion. Uniform nanoparticles of zirconia keep the heat expansion coefficient constant and produce ceramic products at low temperatures, which saves energy consumption. Zirconium oxides, zinc and barium nanoparticles have a better efficiency than their ordinary oxides because of their very small particles, and their high levels of activity. There are several methods for synthesizing these nano-oxides, but the methods used in this project are economically more sound.
Experimental Section
Devices and Appliances Used
Electric furnace manufactured by Exiton company with large compartment, Alumina plant, vacuum pump, Spa bath, X-ray diffractometer X (XRD) Philips PW 3710 mpd control model, scanning electron microscopy equipped with X-ray Spectroscopy (SEM / EDS) Model LEO440i, Spectroscopic transmitted electron microscopy X-ray (TEM / EDS) Philips 420 machine,Elemental analysis (CHN) were performed on Thermo Finnigan Flash 1112EA elemental analyzer. FT-IR spectra were recorded on FT-IR JASCO 460 Spectrophotometer using.
Used Chemicals
Zirconium Silicate, Hydrochloric Acid, Ammonia, Ethanol, Sodium Hydroxide, Potassium Hydroxide. 1,10 –Phenantrolin, Ammonium oxalate monohydrate, ZnCl2, Ethylenediaminetetra- acetic acid, and ammonioum tioacetamid, Ba(NO3)2, Zn(NO3)2 .6H2O ,bidistilled water.
Synthesis of ZrO2
After an hour, plant containing ZrSiO4 was removed from the furnace and immersed into the water in the steel container in molten form. It was mixed with hydrochloric acid 8M gradually. After 3 hours, the acid had an effect on the content of hydrated zirconium at room temperature and formed ZrOCl2. Insoluble SiO2 was isolated from the solution by a filter. A solution of ZrOCl2 is used for purification. 4 ml of concentrated 12-molar HCl was solved in 70 ml distilled water and was gradually added to the solution. The solution is warmed up to a warm pan of 70°C. The contents of the beaker were purified, and the substrate solution was warmed to a volume of 40 ml. The solution was placed at room temperature until it was cooled. By dispensing the solution, the crystals were first separated by 1: 1 water and ethanol mixture and then equilibrated with water and hydrochloric acid, and after washing, the contents were dried. A portion of 3-molar ammonia solution was added to the crystalline medium. Chlorides form ammonium chloride, and Chlorine intrusions are thus eliminated. The best method for removing SiO2 impurities is to perform drying operations with the help of a blue bath that causes the appearance of insoluble silica inside the beaker. If filtration is performed twice, the solution would be completely free of SiO2. A vacuum pump was used to speed up the operation. For heat treatment on the remaining powder, some of the prepared specimen was poured into the alumina plant and transferred to the electric furnace. Since zirconium dioxide is intended to be used to determine the optimal temperature and time for synthesis, experiments were carried out at 800, 850, 900, 950, 1100,1000 0C with a duration of one, two and three hours.
Preparation of Zinc and Barium Nano-oxides
For the preparation of Zinc and Barium Nano-oxides, first, the two-component complexes sample 2 ([Ba (o-phen) 4] [Zn (EDTA)]),sample 3( [Zn(o-phen)4]2 [Ba(SCN)6]) were then heated for one hour in 700 and 800°C temperatures inside the electric furnace to form Zinc and Barium Nano-oxides.In order to prepare these complexes, cationic and anionic sections were synthesized separately using the stoichiometric ratio. Also, the cationic and anionic parts were synthesized in a similar manner to the reference [12-16]. X-ray spectroscopy(fig1)shows observed particles of Zinc and Barium nano-oxides. SEM images (Fig 6 and 7 ) were obtained for samples [Ba(o-phen)4][Zn(EDTA)] and [Zn(o-phen)4]2 [Ba(SCN)6] after the furnace. The particle diameters of this samples were determined after heating by nano-particle analyzer as observed in Fig 8 and 9. From these diagrams and SEM pictures ,the samples’ nanopowder is confirmed.
Synthesis of [Zn(o-phen)4]2 [Ba(SCN)6]
2 mmol of Zn(NO3)2 .6H2O salt was dissolved in 25 ml of twice distilled water and 8 mmol of 1,10 –Phenantroline was dissolved in 25 ml of ethanol, separately and stirred by a magnetic blender for 12 hours in the room temperature. The anionic section was also synthesized. Firstly, 1 mmol Ba(NO3)2 and 6 mmol of NH4SCN were dissolved separately in 20 ml of twice distilled water. This solution was stirred for 12 h. In order to create the final solution, the anionic section was slowly added to the cationic section again and a milky solution was obtained. This solution was put in laboratory temperature until the water vaporized slowly. After a week, a white solid substance was obtained. Elemental Analysis calcd for C102 H64 BaN22 S6 Zn2 :C, 59.53; H, 3.11; N, 14.98; %. Found : C, 58.92; H, 3.22; N, 13.89%. This complex was studied using the FT-IR technique. FT-IR spectra for this complex were recorded at room temperature by dispersing minute amounts of solids in KBr pellets (Fig. 1). The wide peak belongs to the tensile vibration of the N-H in the 3406 cm–. For o-phen, the significant peaks are in the range of 1400-1620 cm-1. The sharp peaks appearing at 729, 845 and 1422cm-1 can be attributed to the C=C, C=N of the py ring [17]. Stretching frequency at 3050 cm-1 is related to the C-H bond in the aromatic ring. Also, the Zn-N peak is observed at the lower area of 500cm-1.
![Figure 1: FT-IR spectrum of [Zn(o-phen)4]3 [Ba(SCN)6] in KBr pellets](http://www.orientjchem.org/wp-content/uploads/2018/05/Vol34No3_Pre_Moh_fig1-150x150.jpg) |
Figure 1: FT-IR spectrum of [Zn(o-phen)4]3 [Ba(SCN)6] in KBr pellets |
Synthesis of [Ba(o-phen)4] [Zn(EDTA)]
To an aqueous solution (20 mL) of ZnCl2. 2H2O (273 mg, 2 mmol), a solution of EDTA salt (744 mg, 2 mmol) in water (20 mL) was added and the mixture was stirred at room temperature for 13 hours. The anionic section was also synthesized . Firstly, 2 mmol BaCl2.2H2O was dissolved in 30 ml twice distilled water and 8 mmol 1,10 –Phenantroline was dissolved in 20 ml of ethanol, separately .This solution was stirred for 13 hours. The final solution was obtained by slowly adding the anionic section to the cationic section again. The final solution was filtered and left for slow evaporation in air. Elemental Analysis calcd for C57 H42 BaN10 O8 Zn : C, 57.16; H, 3.53; N, 11.69; %. Found: C, 56.22; H, 3.37; N, 11.16%. This complex was studied using the FT-IR technique. The peaks observed in the range of 500 to 1500 cm-1 are related to both EDTA and o-phen ligands(Fig 2). The wide peak in the 3446 cm-1 belongs to the O-H. The sharp peaks which appear in the range 1612 cm-1 and 1404 cm-1 are attributed to as(COO–) and s(COO–) of the EDTA ligand, respectively.18-21 The stretching vibration of the C-H bond in the aromatic ring is observed around 3050 cm-1, while the tensile vibrations of the bond of C=N and C=C are at 1400 to 1612 cm-1, respectively. They cannot be seen separately because of the overlap with the adjacent peak.
![Figure 2: FT-IR spectrum of [Ba(o-phen)4][Zn(EDTA)] in KBr pellets](http://www.orientjchem.org/wp-content/uploads/2018/05/Vol34No3_Pre_Moh_fig2-150x150.jpg) |
Figure 2: FT-IR spectrum of [Ba(o-phen)4][Zn(EDTA)] in KBr pellets
|
Results and Discussion
The main objective of most of the nanoscale research is to form new compounds by altering existing materials, which have a uniform powder form and a small particle size that increases their qualities. Producing lighter and stronger materials will reduce costs. In this research, access to technical knowledge for the production of these powders and the answer to scientific questions in this regard are important for our purposes. Recent advances in nano science combined with new methods for studying, fabricating and improving the structure of nanoparticles is mainly due to advances in nanochemistry. Since the methodology used in this project has so far been presented in scientific sources, these studies are important and have been reported for the first time. Therefore, in the experiments, the effect of temperature and reaction conditions on the synthesis of zirconium, zinc and barium oxides has been studied to determine the optimal conditions for the synthesis of these powders. Zinc oxide is a substance with one of the most unique properties. This substance has gained paramount importance in the last century because of its use in power storage devices. By synthesizing zinc oxide nanoparticles, the properties of this material can be improved and thus provide more applications. One of the special properties of nanoparticles on zinc oxide is its high catalytic activity, high chemical stability, ultraviolet absorption, low dielectric constant, and most importantly antimicrobial properties. Zinc oxide nanoparticles are one of the most widely used nanoparticles in industry and medicine, which is being added to its application day to day, so its importance is so high that many researchers are trying to design new methods for the synthesis of these nanoparticles. Zinc oxide nanoparticles are one of the most widely used nanoparticles due to their physical and chemical properties, which has attracted the attention of many researchers. In general, nanoparticles are stable due to their high surface-to-volume ratio and superficial interaction, and are of great interest.
Investigating the Ratio of Zirconia to Hydroxide
To convert ZrSiO4 to ZrO2, it is necessary to melt the initial material with a melting point of 1850°C, which is not economical, but the reaction of zirconium silicate with sodium hydroxide and potassium reduces the melting temperature. Different ratios of sodium hydroxide and potassium hydroxide were used at 900°C, and the most suitable alkali melting compound, using Soda and potash melting aid was determined to be 2-to-1 ratio of the zircon weight. First, the melting aim should be fine-grained with zircon so that their sizes are reduced and become well mixed. Due to the use of microgranular amounts, the effect of the use of ZrSiO4 is to reduce the amount of alkali melting.
Determining the Melting Temperature
After determining the proper composition, in order to select the optimum temperature for the melting of the alkali, the experiments were carried out at 700, 800, 850, 900, 950, 1000, 1100, 1200°C and 2 hours, respectively. The results of this experiment show that the lowest required temperature for melting the alkali is 850°C. In the higher temperature, melting operation is better and faster. However, melting at high temperatue is not economical.
Determining The Melting Time
In order to determine the best shelf-life at optimum temperature of alkali melting, the plant, along with its contents inside the furnace, was placed at different intervals of 60, 70, 80, 90, 100 and 120 minutes. The best lifetime for zirconium melting was determined to be 90 minutes. It is also necessary that the crucible be installed inside the furnace for the first time, because if the furnace reaches the melting point of the material and the plant and its contents is then placed inside the furnace, thermal shock will break the crucible.
Investigation of The Effect of Calcination Temperature and Durability Time
The temperature and durability time of the powder can affect the size of the particles. The results of this study are presented in Table (1).
Table 1: The effect of time and durability on particle size of ZrO2
| Temperature (0C) | durability time (h) | Average particle size(nm) |
| 900 | 2 | 150 |
| 900 | 3 | 320 |
| 1000 | 2 | 340 |
| 1000 | 3 | 500 |
| 1100 | 1 | 300 |
| 1100 | 2 | 680 |
The smallest particles were synthesized at a temperature of 900°C and a durability time of 2 hours. If the temperature and shelf-life are less than that, pure ZrO2 powder is not obtained and if the calcination temperature and the shelf-life are less than the specified value, the particles are synthesized. Therefore, the temperature and duration of the shelf-life will be set to the aforementioned amount. In the fig. 3, the sample X-ray diffraction pattern of X-ray zirconium powders at a cooking of 900°C and a 2-hour shelf-life is compared with X-ray diffraction pattern of X-ray powder syntheses at 1100°C and 3 hours of shelf-life. In both patterns, only the ZrO2 peaks correspond to the PDF02-0733 and PDF36-0420 cartridges, but the existing peaks in the diffusion pattern of the synthesized sample under optimum calcination temperature conditions are clearly wider than the peaks of X-ray synthesized powder at 1100°C and the shelf life of hours. This indicates the finer grain size in these conditions. In order to assure the composition and size of the synthesized grains, they were taken from microscopic images, which are optimally optimized in the SEM image of the zirconium oxide powder. An example of X-ray spectroscopy of the observed particles is also shown in Fig. 5. It can be concluded from these images that the size of the particles is nano-meterical, and their composition is pure zirconium oxide.
 |
Figure 3: X-ray diffraction pattern of zirconium powders has been synthesized (a)at a cooking of 900°C and 2-hours durability time (b) at 1100°C and 3 hours |
 |
Figure 4: SEM image of the Zro2 powder has been synthesized at a cooking of 900°C and a 2-hour durability |
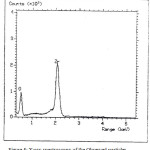 |
Figure 5: X-ray spectroscopy of the Observed particles |
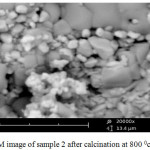 |
Figure 6: SEM image of sample 2 after calcination at 800°C Click here to View figure |
 |
Figure 7: SEM image of sample 3 after calcination at 700°C |
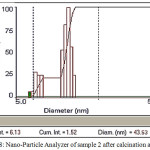 |
Figure 8: Nano-Particle Analyzer of sample 2 after calcination at 800°C |
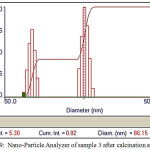 |
Figure 9: Nano-Particle Analyzer of sample 3 after calcination at 700°C |
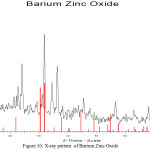 |
Figure 10: X-ray pattern of Barium Zinc Oxide |
Applications of Nanometeric ZrO2
Alumina-zirconium dioxide ceramics have a higher abrasion and abrasion resistance than conventional alumina, as a result of which composite ceramics are used as cutting tool tips. Alumina-zirconia abrasive plates have a higher performance than pure aluminum. To cut industrial materials, especially magnetic tapes, plastic materials and cigarette filters, ZrO2 blades are used. Chemical pumps, sludge pumps and gaskets are made of zirconium ceramics. A pump made from TZP is now commercially available. Zirconia is currently being investigated as a biologically active material instead of worn joints. The high melting point and excellent chemical properties of zirconia designates its use as antifreeze. For this reason, the nozzles and molten melt stoppers and the Sliding Gate Valve, an important component of the casting process equipment, are used. The best material to install in refractory bricks for high temperatures is porous insulating bricks with zirconium stabilized and used in furnace elements. Zircon blocks can be used as unshielded bricks of stabilized zirconia. As a thermal and chemical shield, a special application of this material has been used in the core of nuclear reactor. As a thermal insulation protector, they can increase the performance of the engine and the aircraft. In 1965, the feasibility of making oxygen sensors was commercially registered. Today, the use of oxygen-based zirconium dioxide base is commonly used in applications such as combustion control, atmospheric control in thermal operation furnaces, and as a warning for the amount of oxygen in molten metals. The commonly used zirconia MHD electrodes have been successfully produced with additional CeO2 and Ta2O5 materials. A high quality zirconium is used for more than 99.77% for electronic applications. One of the most important applications of piezoelectric ceramics is the electric-mechanical membrane of the audio, and is widely used to polish the glass as well as to the duct mist. Zirconium dioxide plays a role in germination in glass and ceramics. ZrO2-based dyestuff agents are widely used in tile industries, dining utensils and sanitary products.
Conclusion
The current research was carried out with the aim of synthesizing ZrO2, BaO and ZnO powder in an economically reasonable way. Zirconia and barium zinc oxide nanopowders were studied by XRD ,SEM considering these diagrams and SEM pictures the nanopowder samples are confirmed. In the synthesis of ZrO2 ,the most suitable alkali melting compound, using NaOH and KOH was determined to be 2-to-1 ratio of the zircon weight. In optimum conditions, the smallest particles in calcination temperature of 900°C and durability time of two hours was synthesized in furnace.
Acknowledgement
We are gratefully to the collaboration of the Payame Noor University and Sistan & Baluchestan University in this work.
References
- Choi C.J.; Dong X.L.; Kim B.K. Seripta meter. 2001, 44, 2225-2229.
CrossRef - Luan W.; Gao L.; Guo J. Nanostract. Mater. 1998, 10, 1119-1125.
CrossRef - Sergeev G.B., Nanochemistry. Elsevier. 2006,5, 175-200.
CrossRef - Guo S.; Wang E. Noble metal nanomaterials. Nanotoday. 2009, 933, 547-555
- Xia Y.; Xiong Y.; B Lim.; Skrablak E.S.Angewandate chemie International Edition. 2009, 48(1) ,60-103.
- Alexander S.; Hellemans L.; Marti O.; Schnier J.; Elings V.; Hansma P.K.; Longmiro M.; Gurley J. Journal of applied Physics 1989, 65(1), 164-167.
CrossRef - Salamanca-Buentello F.; Persad D.L.; Court E.B.; Martin D.K.; Daar A.S.; Singer P.A. nanotechnology and developing word. Plos Medincine.2005, 2(5), 383-386
- Service R.F. Nanochemistry, nanoparticles, trojan horses gallop from the lab into the clinic. Scince. 2010, 330(6002), 314-315.
CrossRef - Hung H.C.; Baru S.; Sharma G.; Dey S.K.; Reg K. Jornal of Controlled Releasse. 2011, 155, 344-357.
CrossRef - Wiess J.; Takhistov P.; Mclemeat D.J. Journal of Food Science. 2006, 71, 107-116.
CrossRef - Salavati Niyasari M.; Fereshteh Z. Nanochemistry. Elmo-danesh. Tehran,2008, 25-40.
- Mao L.; Wang Y., Qi Y.; Cao M.; Hu C. Journal of Molecular Structure. 2004, 688, 197–201.
CrossRef - Uçar I, Karabulut B.; Bulut A.; Buyukgungr O Journal of Molecular Structure. 2017, 834–836 , 336–344
- Wood D.L.; Rabinovich E.M. Journal of Non-Crystalline Solids. 1989, 107, 199–211.
CrossRef - Yamame M.; Klein L.C. Noyes Publications New Jersey. 1989, p. 200.
- Cordoba G.; Arroyo R.; Fierro J.L.G. Viniegra M.J. Journal of Solid State Chemistry. 1996, 123, 93–99.
CrossRef - Vargova Z.; Zeleoak V.; Cisaova I.; Gy ryova K. Thermochimica Acta.2004, 423.
- Nakamoto K. 5th ed. Part B. J. Wiley: New York, 1997.
- Gargallo-Esteban M. F.; Vilaplana-Serrano R.; González-Vilchez F. Spectrochim. Acta 1987, 43A, 1039.
- Busch D. H.; Bailar j.r. J. C.; J. Am. Chem. Soc. 1953, 75, 4574.
CrossRef - Busch D. H.; Bailar j.r. J.C. J. Am. Chem. Soc. 1956, 78, 716.
CrossRef

This work is licensed under a Creative Commons Attribution 4.0 International License.









