Synthesis and thermal characterization of mono and dicationicimidazolium-pyridinium based ionic liquids
Reza Fareghi-Alamdari1, Faezeh Ghorbani–Zamania1,*, Marzieh Shekarriz2
1Faculty of Chemistry and Chemical Engineering, Malek - Ashtar University of Technology, Tehran, Iran.
2Research Institute of Petroleum Industry, West Blvd. Azadi Sports Complex, Tehran, Iran.
* Corresponding authors E-mail: faezehzamanie@yahoo.com
DOI : http://dx.doi.org/10.13005/ojc/310265
Article Received on :
Article Accepted on :
Article Published : 10 Jun 2015
A series of monocationic and dicationic ionic liquids, in which each ionic liquid is associated with dicyanamide anion, are synthesized. The molecular structures of these compounds were identified by 1H-NMR, FT-IR and CHN analysis. Thermal behaviors of these ionic liquids were also investigated by thermogravimetry (TGA). The results suggested that all new ionic liquids have reasonable thermal stabilities, however, dicationic ionic liquids show more thermal stability than monocationics.
KEYWORDS:liquids; dicationic ionic liquids; dicyanamide anion; imidazolium-based ionic liquids; Thermal properties
Download this article as:| Copy the following to cite this article: Fareghi-Alamdari R, Ghorbani–Zamania F, Shekarriz M. Synthesis and thermal characterization of mono and dicationicimidazolium-pyridinium based ionic liquids. Orient J Chem 2015;31(2). |
| Copy the following to cite this URL: Fareghi-Alamdari R, Ghorbani–Zamania F, Shekarriz M. Synthesis and thermal characterization of mono and dicationicimidazolium-pyridinium based ionic liquids. Available from: http://www.orientjchem.org/?p=9174 |
Introduction
Ionic liquids (ILs) are polar compounds of poorly coordinating ions which are liquid below 100 °C and have potential as a clean replacement for common volatile organic compounds (VOCs) in chemical process. Compared to molecular organic compounds, ILs exhibit several unique properties such as low vapour pressure, non-flammability,liquidity over wide temperature range, high thermal and chemical stability, ionic conductivity and structural designability [1, 2]. These unique properties of ILs open up a widespread range for series of applications including catalysis [3], organic synthesis [4], separation and analysis [5], electrochemistry [6], energy technology [7], as well as many others. For general understanding of these materials it is of importance to determine their thermodynamic and physicochemical properties as well as predict properties of unknown ionic liquids to optimize their performance and increase their potential future application areas. For many applications, a major goal is that IL maintains its chemical structure under the condition of use. As known, one of the importance characteristics of ionic liquids is that their physical and chemical properties can be tailored to specific application by adjusting or tuning the structure and functionality of the cations and anions [8]. Changes in physicochemical and thermal properties of ILs by an atom substitution are not very large compared to whole ion exchange, while the atom replacement could be useful for sophisticated control for ILs properties. To improve thermal stability, a series of ionic salts, which consists of two mono-cations are linked together to form a dication that is charge balanced by two separate mono anions has been developed and the salts are termed dicationic ILs [9, 10].
Recently, synthesized and characterization of several ammonium-based dicationic phosphate salt were reported by Engel and co-workers [11, 12]. Ohno and co-workers have prepared some imidazolium-based dicationic ILs [13].
Interestingly, dicationic ILs show higher thermal stability than monocationic ILs and melting points higher than 100 °C [9]. For decreasing melting points, some asymmetric dicationic ILs whose cation has two different moieties have also been synthesized by several groups [14, 15]. Nevertheless, there is no doubt that deeper knowledge of physicochemical and thermal properties of dicationic ILs is necessary to find the unique and general features and control their properties. In view of the fact that the dicationic ILs are more thermal stable than the dicationic, this paper offers the synthesis and characterization of new asymmetricdicationic ILs as well as monocationic ILs. Furthermore, the thermal behaviours of the synthesized ILs were investigated by thermal gravimetric analysis (TGA). We also compare the thermal stability of prepared mono and dicationic ionic liquids with each other.
Experimental
Reagents and instruments
All of the solvents and chemicals were purchased from Sigma-Aldrich and were used without further purification. All synthesized compounds were characterized by 1H-NMR spectroscopy using BrukerDRX – 300. FT-IR spectra were obtained on a Nicolet 800 instrument. Elemental analyses were obtained using Heraeous CHN analyzer (Germany) instrument. Thermal gravimetric analyses (TGA) were recorded using a thermal analysis instrument Perkin Elmer (Pyris Diamond model). Runs were carried out under an inert atmosphere of nitrogen. Initial mass introduced in the pan was set in 10 mg. Dynamic measurements were conducted at 25 to 600 °C.
Ionic liquid synthesis
The general procedure for the synthesis of mono and dicationic ILs is shown in Scheme 1.
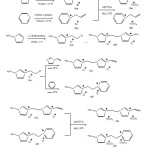 |
Scheme1: Synthesis routes of mono and dicationic ionic liquids Click here to View scheme |
General reaction for synthesis monocationic ionic liquids
To a vigorously stirred solution of 1-methylimidazole or pyridine (25.0 g, 310 mmol) in toluene (125 cm3) at 0oC was added 3-bromo-1-propene (29.86 cm3, 345mmol). The solution was heated to reflux at ca. 110oC for 24 hours, after which the toluene was decanted and the remaining viscous oil/semi-solid re-crystallized from acetonitrile and then repeated re-crystallized from ethylacetate to yield a brown viscous liquids, which was dried in vacuo to give 1a and 1b in approximately 93 % yields.
1-allyl-3-methylimidazolium bromide (1a)
1H-NMR (300 MHz, D2O): δ 8.65 (s, 1H), 7.43 (dd, 1H), 7.36 (dd, 1H), 6.11-5.78 (m, 1H), 5.35 (dd, 1H), 5.19 (dd, 1H), 4.69 (d, 2H), 3.61 (s, 3H). IR (KBr, ν/cm-1): 3426, 3145, 3032, 1668, 1573, 1268, 1112, 949. Anal.Calcd. For C7H11N2Br: C, 41.37; H, 5.41; N, 13.79%; found: C, 40.73; H, 6.65; N, 13.15%.
1-allyl-pyridinium bromide (1b)
1H-NMR (300 MHz, D2O): δ 8.75 (d, 2H), 8.39 (t, 2H), 7.91 (t, 1H), 5.7 (m, 1H), 5.46 (dd, 1H), 5.11 (dd, 1H), 2.01 (d, 2H). IR (KBr, ν/cm-1): 3129, 3056, 2936, 2874, 1633, 1487, 1270, 1172, 936. Anal.Calcd. For C8H10NBr: C, 48.00; H, 5.00; N, 7.00%; found: C, 47.73; H, 4.65; N, 7.15%.
General procedure for the synthesis of dicationic ionic liquids
Compound 1c was prepared by heating 1-methylimidazole (10.0 g, 122 mmol) and 1, 2-dichloroethane (50.0 g, 505 mmol) to reflux for 3 h. The oily precipitate was separated, washed with ethyl acetate (30 mL) and dissolved in a mixture of isopropanol (10 mL) and acetone (30 mL). This solution was kept in a freezer overnight, to ensure the crystallization of the by-product 1,2-bis (3-methylimidazolium-1-yl) ethane dichloride. The remaining solution was separated from the crystals and was evaporated, giving the desired 1-methyl 3-(2-chloroethyl) imidazoliumchloride as pale amber oil, sufficiently pure for further work (34 % yield).
Compounds 1e and 1f were prepared by reacting compound 1c with a slight molar excess of 1-vinylimidazole and pyridine for 24 h at room temperature, respectively. The precipitates were filtrated, washed, and purified by ethyl ether to afford the corresponding salts (93 % yield).
1-methyl 3-(2-chloroethyl) imidazolium chloride (1c)
1H-NMR (300 MHz, D2O): δ 8.87 (s, 1H), 7.47 (d, 1H), 7.44 (d, 1H), 4.62 (t, 2H), 3.88 (s, 3H), 3.80 (t, 2H). IR (KBr, ν/cm-1): 3442, 3145, 2936, 1572, 1465, 1309, 1169, 622. Anal.Calcd. For C6H10N2Cl2: C, 39.78; H, 5.52; N, 15.47%; found: C, 40.23; H, 4.82; N, 15.15%.
1-[2-(1-vinylimidazolium)-yl-ethyl]-3-methylimidazolium dichloride (1e)
1H-NMR (300 MHz, D2O): δ 9.03 (s, 1H), 8.69 (s, 1H), 7.72 (t, 1H), 7.52 (t, 1H), 7.42 (t, 1H), 7.37 (d, 1H), 7.05 (m, 1H), 5.72 (dd, 1H), 5.34 (dd, 1H), 4.24 (m, 4H), 3.79 (s, 3H). IR (KBr, ν/cm-1): 3442, 3143, 3093, 1632, 1572, 1459, 1314, 1160, 946. Anal.Calcd. For C11H16N4Cl2: C, 48.00; H, 5.81; N, 20.36%; found: C, 49.32; H, 5.82; N, 20.62%.
1-(1-pyidinium-yl-ethyl)-3-methylimidazolium dichloride (1f)
1H-NMR (300 MHz, D2O): δ 9.00 (d, 2H), 8.66 (t, 1H), 8.50 (s, 1H), 8.16 (t, 2H), 7.80 (dd, 1H), 7.59 (dd, 1H), 3.70 (s, 3H), 3.32 (t, 2H), 2.9 (t, 2H). IR (KBr, ν/cm-1): 3429, 3146, 2955, 1634, 1585, 1365, 1281, 1161, 832. Anal.Calcd. For C11H15N3Cl2: C, 50.76; H, 5.76; N, 16.15%; found: C, 49.73; H, 5.82; N, 15.92%.
General procedure for anion exchange reaction
Silver dicyanamide was prepared by mixing equal molar amounts of silver nitrate and sodium dicyanamide in aqueous solution followed by filtration. Compounds 2a and 2b were prepared by performing anion exchange for compounds 1a and 1b with 1mmol silver dicyanamide, while compounds 2e and 2f were synthesized by reacting 1e and 1b with 2mmol silver dicyanamide in deionized water for 24 h at room temperature (91 % yield).
1-allyl-3-methylimidazolium dicyanamide(2a)
1H-NMR (300 MHz, D2O): δ 8.63 (s, 1H), 7.45 (dd, 1H), 7.36 (dd, 1H), 6.10-5.77 (m, 1H), 5.37 (dd, 1H), 5.16 (dd, 1H), 4.68 (d, 2H), 3.60 (s, 3H). IR (KBr, ν/cm-1): 3431, 3144, 3084, 2233, 2194, 2135, 1625, 1573, 1312, 1167, 947. Anal.Calcd. For C9H11N5: C, 57.14; H, 5.82; N, 37.03%; found: C, 56.73; H, 6.11; N, 37.15%.
1-allyl-pyridinium dicyanamide(2b)
1H-NMR (300 MHz, D2O): δ 8.68 (d, 2H), 8.36 (t, 2H), 7.95 (t, 1H), 5.74 (m, 1H), 5.45 (dd, 1H), 5.06 (dd, 1H), 1.94 (d, 2H). IR (KBr, ν/cm-1): 3424, 3124, 3044, 2966, 2236, 2194, 2134, 1630, 1582, 1486, 1310, 950. Anal.Calcd. For C10H10N4: C, 63.49; H, 5.29; N, 29.62%; found: C, 63.73; H, 4.65; N, 29.15%.
1-[2-(1-vinylimidazolium)-yl-ethyl]-3-methylimidazolium dicyanamide(2e)
1H-NMR (300 MHz, D2O): δ 9.28 (s, 1H), 8.68 (s, 1H), 7.70 (t, 1H), 7.50 (t, 1H), 7.41 (t, 1H), 7.38 (d, 1H), 7.05 (m, 1H), 5.71 (dd, 1H), 5.34 (dd, 1H), 4.21 (m, 4H), 3.73 (s, 3H). IR (KBr, ν/cm-1): 3392, 3142, 3072, 2231, 2196, 2143, 1636, 1567, 1456, 1306, 1165, 952. Anal.Calcd. For C15H15N9: C, 56.07; H, 4.67; N, 39.25%; found: C, 56.32; H, 4.82; N, 39.42%.
1-(1-pyidinium-yl-ethyl)-3-methylimidazolium dicyanamide(2f)
1H-NMR (300 MHz, D2O): δ 8.98 (d, 2H), 8.65 (t, 1H), 8.43 (s, 1H), 8.17 (t, 2H), 7.81 (dd, 1H), 7.63 (dd, 1H), 3.65 (s, 3H), 3.20 (t, 2H), 2.86 (t, 2H). IR (KBr, ν/cm-1): 3427, 3143, 2957, 2195, 2134, 2042, 1668, 1317, 1164, 1088, 828. Anal.Calcd. For C15H15N9: C, 56.07; H, 4.67; N, 39.25%; found: C, 55.73; H, 5.10; N, 38.92%.
Result and discussion
Identification of the synthesized compounds
Molecular structures of all synthesized compounds in this study were characterized via FT-IR, 1H-NMR and CHN analysis. All the data of characterization are in accordance with the expected compositions and structures.
Thermal stability
The TGA curves recorded for the synthesized monocationic and dicationic ILs are illustrated in Figure 1.The thermal decomposition temperatures (Td) estimated from these curves are shown in Table 1.
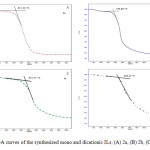 |
Figure1: TGA curves of the synthesized mono and dicationic ILs; (A) 2a, (B) 2b, (C) 2e, (D) 2f. Click here to View figure |
As can be seen from Figure 1, salts that contain imidazole group (2a and 2e) are thermally more stable than those containing the pyridine ring (2b and 2f). This may be due to the fact that the imidazolium groups have better positive charge dispersion than does the pyridine group, which give them a stronger attraction to anions [16].
We also found that the dicationic ILs are substantially more thermal stable than that monocationic ILs (Figure 2). It is obvious that the decomposition temperature of the dicationic ILs are approximately 20 °C to 40 °C higher than that the monocationic ILs which have identical anions or even substituted alkyl. This higher thermal stability in the dicationic ILs compared with monocationic would be related to higher liquid density in the dicationicILs [17]. Thus, the dicationic ILs are suitable for utilization at higher temperature condition and application related to local heating [18].
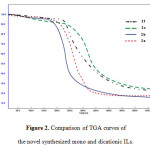 |
Figure2: Comparison of TGA curves of the novel synthesized mono and dicationic ILs. Click here to View figure |
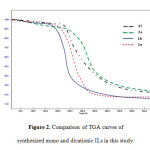 |
Figure2: Comparison of TGA curves of synthesized mono and dicationic ILs in this study. Click here to View figure |
Comparison of thermal stability of mono and dicationic ILs
Thermal stability of ILs in this study compared with the some values reported in the literature. The results are collected in Table 1. With regarded to anion effect, data in Table 1, indicates that the thermal stability of ILs may be related to the nucleophilicity of the anions which used in ILs structures. The thermal stability of ILs increases with increasing the nucleophilicity of the anions [19].Table 1 also reveals that, with regard to the cation effect, ILs which composed of imidazolium moiety have higher thermal stability than those consist of pyridine rings because of more charge dispersion and stronger anion attraction. Moreover, it is reported that decomposition in imidazolium-based ILs occure at C-N bands [20]. According to this fact, a smaller free volume and higher viscosity could works effectively for the recombination. Also, a small displacement of ion’s vibrations due to small free volume could suppress the decomposition of the ions and increase thermal stability.
 |
Table1: comparison of thermal stability values of different ILs. Click here to View table |
Conclusion
Dicationic and monocationic ILs that contain imidazole and pyridine moieties were successfully synthesized. All prepared ILs exhibited good thermal stabilities up to 200 °C. Compared to monocationic ILs, dicationic ILs have higher thermal stability due to higher degree of positive charge dispersion which give them a stronger attraction to anions. Moreover, when a fair comparison (same anion, alkylene linker and alkyl group) was made, the ILs that contain imidazolium cationic moiety have higher thermal stability than those that contain pyridiniumrings. The imidazolium-based ILs show higher thermal stabilities because of more positive charge dispersion, smaller free volume and higher viscosity.
Acknowledgements
The authors are grateful to Malek – Ashtar University of Technology for supporting this work.
References
- OhnoH. Electrochemical Aspects of Ionic Liquids; Wiley: Hoboken, NJ, (2005).
- Letaief, S.; Detellier, C. J. Mater. Chem. 2007, 17, 1476-1484.
- Godajdar, , B. M.; Kiasat, A. R.; Ezabadi, A.Orient. J. Chem.2015, 31, 483-487.
- Johari, N. L. S.; Hassan, N. H.; Hassan, N. I. Orient. J. Chem.2014, 30, 1191-1196.
- Han, X.; Armstrong, D. W. Acc. Chem. Res. 2007, 40, 1079-1086.
- Silvester, D. S. Analyst.2011, 136, 4871-4882.
- Wishart, J. F. Energy Environ. Sci. 2009, 2, 956-961.
- Kulkarni, P. S.; Afonso, C. A. M. Green Chem. 2010, 12, 1139-1149.
- Payagala, T.; Huang, J.; Breitbach, Z.S.; Sharma, P.S.; Armstrong, D.W. Chem. Mater. 2007, 19, 5848-5850.
- Zeng, Z.; Phillips, B.S.; Xiao, J.; Shreeve, J.M. Chem. Mater. 2008, 20, 2719-2726.
- Lall, S.; Behaj, V.; Mancheno, D.; Casiano, R.; Thomas, M.; Rikin, A.; Gaillard, J.; Raju, R.; Scumpia, A.; Castro, S.; Engel, R.; Cohen, J. I. Synthesis.2002, 1530-1539.
- Wishart, J. F.; Lall-Ramnarine, S. I.; Raju, R.; Scumpia, A.; Bellevue, S.; Ragbir, R.; Engel, R. Radiat. Phys. Chem.2005, 72, 99-104.
- Ito, K.; Nishina, N.; Ohno, H. Electrochim. Acta.2000, 45, 1295-1298.
- Wang, R.; Gao, H.; Ye, C.; Shreeve, J. M. Chem. Mater.2007, 19, 144-152.
- Payagala,T.; Huang, J.; Breitbach, Z. S.; Sharma, P. S.; Armstrong, D. W. Chem. Mater. 2007, 19, 5848-5850.
- Crosthwaite, J.M.; Muldoon, M.J.; Dixon, J.K.; Anderson, J.L.; Brennecke, J.F. J.Chem. Thermodyn.2005, 37,559-568.
- Shirota, H.; Mandai, T.; Fukazawa, H.; Kato, T. J. Chem. Eng. Data.2011, 56, 2453–2459.
- Huang, K.; Han, X.; Zhang, X.; Armstrong, D. W.Anal. Bioanal. Chem.2007, 389, 2265-2275.
- Hsieh, Y. N.; Ho, W. Y.; Horng, R. S.; Huang, P. C.; Hsu, C. Y.; Huang, H. H.; Kuei, C. H. Chromatographia. 2007, 66, 607-611.
- Ohtani, H.; Ishimura, S.; Kumai, M.Anal. Sci. 2008, 24, 1335-1340.
- Chang, J.C.; Ho, W.Y.; Sun, I.W.; Chou, Y.K.; Hsieh, H.H.; Wu, T.Y. Polyhedron. 2011, 30, 497–507.

This work is licensed under a Creative Commons Attribution 4.0 International License.









