Abstract
Synthesis of Weinreb and their Derivatives (A Review)
Maher Khalid*, Shireen Mohammed and Amin Kalo
DOI : http://dx.doi.org/10.13005/ojc/360201
Abstract:
Due to the largely and an efficient usage of Weinreb amides or N-methoxy-N-methylamides as are remarkable intermediate in the organic synthesis field, the recent review paper provides a considerable development literature survey on the Weinreb amides synthesis. The direct transformation of carboxylic acids,acid chlorides, and esters to aldehydes or ketones employing organometallic reagents do not lead in high yields, since the high reactivity of ketone intermediates toward the organometallic reagents. While, the conversion to the appropriate Weinreb Amides, followed by treatment with the organometallic regent, result the stable expected ketones as the stable initial adduct toward further reactions. Furthermore, Weinreb amides undergo nucleophilic addition and produce a unique and steady five-membered cyclic intermediate which protects the over-addition, leading to a serious transformation.
Grapic Abstract
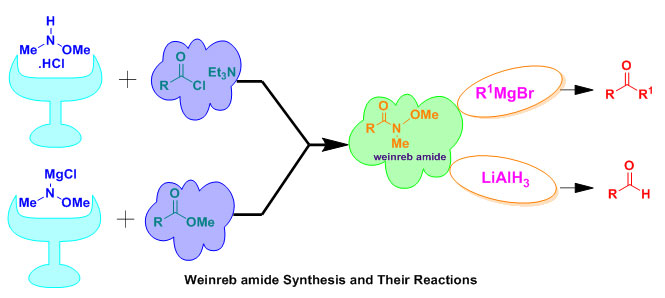
Organometallic Reagents; Weinreb Amides; Weinreb Benzamide; β-Trifiuoromethylated Enaminones; α-Amino Aldehydes.
Back to TOC









