Ricinus Communis Activated Charcoal Preparation, Characterization and Application for Methyl Red Adsorptive Removal
1Department of Chemistry, Lrg Government Arts College for Women, Tirupur-04, Tamil Nadu. India.
2Department of Chemistry, Karpagam Academy of Higher Education, Coimbatore-21, Tamil Nadu. India.
3Hindusthan College of Engineering and Technology, Coimbatore-21,Tamil Nadu. India.
4Sri Eshwar College of Engineering, Kinathukadavu, Coimbatore-02, Tamil Nadu. India.
Corresponding Author E-mail: Ssnilasri@Yahoo.Co.In
DOI : http://dx.doi.org/10.13005/ojc/380114
ABSTRACT:The phosphoric acid activated Ricinus communis stem carbon (PRCS) is used to degrade methyl red dye from aqueous solution under solar light irradiation The studied Physiochemical properties, surface morphology, elemental composition and crystalline nature of PRCS are reported. The optimum experimental conditions are fixed by optimizing the experimental parameter such as contact time, pH, carbon doses and dye concentration. The maximum degradation efficiency of MR by UV spectroscopy found to be 82.79 % at pH 3, 0.25 gm of PRCS, 90 min irradiation time and dye concentration of 20 ppm. Adsorptive and photo catalytic degradation of dye explained by isotherm and kinetic studies. From the results it is clearly evident that PRCS could be used as an ecofriendly photo catalyst for the removal of dyes from waste water.
KEYWORDS:Activated charcoal; Degradation; Methyl red dye; PRCS; Ricinus communis
Introduction
Textile effluent contain large amount of synthetic dyes. These dyes are mixed with soil and water resource that are carcinogenic, toxic and neurotoxic in nature1. Discarding the dyes in water source is a difficult one because they are very sturdy and they are very soluble in water. If the waste waters are not managed properly, they will harmful to the environment. Dye effluent containing variety of dyes. Methyl red used in textile dyeing, paper printing and as an indicator in acid base titrations. Discharge of Methyl red from various industries cause harmful effects to human beings, plants, animals and the environment. It causes skin irritation and skin sensitization. If inhaled it damages the central nervous system, digestive path irritation, kidney failure and severe depression. In animal it causes severe effect in reproductive and fetal.
The methods for removal of dyes are oxidation/precipitation, nano filteration, electrocatalytic separation, photochemical degradation, degradation using solar light and adsorption2-4. Among these photo degradation is the efficient, sustainable method for the removal of dyes from waste water. The solar energy is the potential energy source which is utilized for the degradation of dyes. Nowadays, a several number of adsorbents and photocatalysts have been used for dye degradation. Activated charcoal is more effective because of their surface area, and high capacity5. In recent years the agricultural waste is used for preparing activated charcoal and used as a photocatalyst for the removal of dyes. Some examples for agricultural waste was employed for adsorption of dyes such as waste orange peel6, wheat straw7, saw dust8, barely husk9, and water melon rinds10, lime peel11. In this study Ricinus communis 12stem is used and as a photocatalyst. Ricinus communis has the potential for phytoremediation of distillery waste13. The endemic of the plant is India and planted throughout the country in gardens and fields and also breed wild in waste place14-15.
The activation process of a photocatalyst is carried out by either physical or chemical activation process. The properties of the activated carbon can be differed by the precursor, the procedure and process condition. These can perturb the pore sizes, the shape of the pores and their surface16. The carbon yield is too and the temperature employed in chemical activation is lesser than that of physical activation17. In this work Activated charcoal is prepared from Ricinus communis stem (PRCS) by chemical activation method. The chemical activation is processed by using phosphoric acid. The bio adsorbent is used to degrade Methyl red dye from aqueous medium and textile waste water under solar light irradiation. The physiochemical properties of adsorbent were determined18. The kinetic studies were performed. Freunlich, Langmuir, Tempkin, D-R isotherm were investigated. Column studies, UV spectroscopy are used to know MR degradation on PRCS in textile waste water. The surface morphology of before degradation of Methyl red dye, after degradation of methyl red dye on PCRS activated carbon was reported by EDAX, XRD, SEM. The functional group analysis of Ricinus communis stem was identified by FTIR spectroscopy. The importance of this present work is to investigate the Photo catalytic degradation efficiency of PCRS along with isotherm and kinetic models.
Experimental
Material and Methods:
Raw material and chemicals
Ricinus communis stem was collected near Saravanampatti, Coimbatore, Tamilnadu. It was washed with water thoroughly. It was dried, powered and stored in air tight container. NaOH, H3PO4, Methyl red were purchased from Merck Company.
Preparation of Phosphoric acid activated Ricinus communis stem (PRCS)
6 g of Ricinus communis stem powder mixed with 12ml of phosphoric acid thoroughly and kept in a magnetic stirrer for 24 hour agitation. The resultant mixture is heated in a hot air oven for 4 hours, after 0.5M NaOH is added in a room temperature and the whole mixture is kept for 24 hours. Finally the product is washed, filtered and dried in a hot air oven at 100◦ C for 5 Hours.
Characterization
The physiochemical characteristics are reported. The surface analysis of the adsorbent was determined by SEM, EDX. FTIR spectroscopy was used to find out the functional group where present in the adsorbent. UV spectroscopy was employed to detect the biodegradation of the adsorbent. XRD spectroscopy was applied to find out elemental composition of the adsorbent.
Photo catalytic studies
The photocatalytic degradation of Methyl red onto PRCS is studied by taking 50 ml of different dye concentration in a series of 250ml conical flasks with fixed PRCS dose, the mixture is stirred and agitated in a bench shaker for 10-15 minutes for the equilibrium between PRCS and adsorbate solution. After equilibrium, the whole content is exposed to sunlight irradiation. The absorbance is measured at regular interval using UV –VIS spectrophotometer 119. The percentage of degradation efficiency is calculated by the following equation
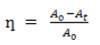
Where A0 is the initial absorbance of the dye and At is the absorbance of dye after degradation 19.
Results and Discussion
Physiochemical Characterization
The physico chemical properties of PRCS were given in Table 1. The higher yield and iodine value indicated that increasing the adsorption ability of the adsorbents20. Acidity of adsorbent surface and basicity of adsorbrnt surface was 4.30 mmol / g, 2.95 mmol/g. Acidity and basicity were calculated by Boehm titration method21. Boehm titration investigated about the basic and oxygenated acidic surface groups on activated carbon21. From this method we conclude that the activated carbon containing phenyl carboxyl groups. This is confirmed by the FTIR analysis results. From the above results t the adsorbent (PRCS) could be employed as an efficient material for the degradation of dyes from waste water.
Table 1: Physiochemical Characterization of PRCS
|
Parameter |
PRCS |
|
Yield |
70% |
|
Moisture content |
5.81% |
|
Ph |
4.82 |
|
Acidity of the Surface |
4.30 mmoles/gm |
|
Basicity of the surface |
2.95 mmoles/gm |
|
pHzpc |
3.6 |
|
Boehm titration Basic sites |
2.30m.eq/gm |
|
Phenolic and Carboxylic groups |
1.13 |
|
Carboxyl groups |
0.73 |
|
Iodine value |
1876.25 mg/g |
Surface Characteristics
Scanning electron microscopic study of PRCS
The physical morphology of the Ricinus communis stem was observed at 10µm magnification. The SEM analysis was done by using the instrument JSM 6390 model. The micrograph (Fig. 1) showed that during carbonisation of the Ricinus communis stem permeated with H3PO4, the evaporative matter develops high pressure, which detonates the integral structure of the particle and generates holes on the surface of the carbon, and also causes the evaporation of acids. During carbonization leaving the area free that was formally occupied by the acids22.
 |
Figure 1: SEM image of PRCS. |
Energy dispersive X-ray Spectra (EDX)
In this, EDX spectrum was utilized to confirm the presence of O, Na, C, Ca, K, Zn, P, Cl, Al, S and Si in the adsorbent. The presence of phosphorous in PRCS was due to the impregnation of Ricinus communis stem with H3PO423. The composition of PRCS is given in the Table 2. EDAX spectra of PRCS are displayed in the Fig. 2.
 |
Figure 2: EDX spectrum of PRCS. |
Table 2: Composition of PRCS
|
Compound |
PRCS (Mass%) |
|
C |
72.62 |
|
O |
26.21 |
|
P |
1.06 |
|
Ca |
0.11 |
|
Na |
— |
|
Al |
— |
|
K |
— — |
|
Cl |
X-ray Refractory Diffraction (XRD)
The XRD pattern of PRCS was shown in Fig. 3. The resultant peaks only have broad peaks and no sharp peak. So this study confirmed the amorphous structure of the adsorbent material PRCS24.
 |
Figure 3: XRD spectra of PRCS. |
Fourier Transform and Infrared Spectroscopy (FTIR)
Functional groups in PRCS was analysed by using FTIR shown in Fig. 4. Using Jasco 460 plus spetrophotometer in the spectral range of 4000-400cm-1 for this studies. The frequency range at 3773.89 cm-1 indicated that the appearance of -OH stretching of phenols and alcohols. The peak at 3524.10 cm-1, 3332.17 cm-1 confirmed that the existence of hydroxyl ion, -OH stretching frequency. The frequency at 1713.83 cm-1, 1510.35 cm-1 shows the presence of C=O stretching, C-O stretching in carbonyl, carboxylic acid and lactones. The frequency around1325.15 cm-1, 1051.25 cm-1,589.28 cm-1 confirmed the C-O vibration, stretching vibration of -OH groups, halide groups.
 |
Figure 4 : FTIR image of PRCS. Click here to View figure |
Photocatalytic studies for the degradation of MR onto PRCS
Effect of contact time
The percentage degradation was found to increase with increasing contact time and then it becomes stable after attainment of equilibrium time as so the maximum degradation achieved at 120 minutes for PRCS. This may be due to the fact that initially surface of adsorbent sites were highly vacant and solute concentration was high, and after the equilibrium time there was no change in the degradation of MR onto PRCS adsorbent, because the active sites are lesser in the surface of the adsorbent at this stage. The maximum degradation efficiency of MR onto PRCS was 82.79%. The effect of contact time for the degradation of MR onto PRCS is shown in the Fig. 5.
 |
Figure 5: Effect of contact time for the degradation of MR onto PRCS |
Effect of solution pH
For this work, 50 ml of 20ppm adsorbate solution, 0.2g of PRCS was taken in the series of conical flask with pH range varying from 2-9 and the whole content kept in a bench shaker for 10 minutes to attain the equilibrium condition25. After the attainment of equilibrium the mixture was exposed to direct sunlight irradiation for the degradation of MR. The absorbance were taken with regular time interval. The degradation efficiency of MR onto PRCS was given in Fig. 6. Maximum degradation of MR dye (82.79%) occurs at the pH of 3, when increasing the solution pH degradation of dye degreases gradually. This is due to the fact that the effect of pH depends on zero point charge of the adsorbent PRCS. The zero point charge of PRCS was 3.6, if the solution pH was below the zero point charge if favours anionic dye degradation since the charge of the composite was positively charged(H+) and if the solution pH was below the zero point charge if favours cationic dye degradation because PRCS was negatively charged(OH–)23
 |
Figure 6: Effect of pH for the degradation of MR onto PRCS |
Effect of adsorbent dose
The effect of adsorbent dose on the degradation of MR dye was investigated and shown in the Fig. 7. There was an increase in degradation efficiency of MR dye while increasing for the dose of PRCS from 0.05g to 0.25g at pH 3 and the contact time of 120 minutes. Furthermore addition of adsorbent dose did not show appreciable change in the degradation efficiency of MR. It was confirmed with Fig. 7.
 |
Figure 7: Effect of PRCS dose for the degradation of MR |
Effect of MR dye concentration
The effect of initial dye concentration of MR onto PRCS was studied using initial dye concentration ranges from 20ppm-140ppm at pH 3 and the contact time is 120 minutes. The degradation efficiency of MR onto PRCS was decreased from 82.79% to 23.14%. The result was given in the Fig. 8, which indicates the reduce in dye degradation with increase in dye concentration.
 |
Figure 8: Effect of initial dye concentration of MR onto PRCS. |
Conformation analysis for the degradation of MR onto PRCS adsorbent
The Ultraviolet-Visible(UV-Vis) spectra for the decay of MR onto PRCS were shown in Figs.9 and 10. The percentage of degradation of MR was 82.79% onto PRCS. The decolourization and the degradation of MR were confirmed by using UV –Visible spectral analysis. Examination of spectral lines of dyes indicated that the absorbance peaks at initial of the dyes drastically reduced when the contact time increases. The effectiveness of the dye decolourization was connected with the dye chemical structure, molecular weight and the presence of functional groups. Results obtained reveal colour removal by the analysis isolate due to the biodegradation of the dyes rather than adsorption.
 |
Figure 9: UV-Spectra of MR onto PRCS |
 |
Figure 10: Before and after treatment of MR onto PRCS |
Degradation of Textile effluent using PRCS and charcoal
The degradation process was carried out using PRCS and charcoal for the textile effluent which was collected from Mangalam, Tirupur District. The degradation process was investigated under direct sunlight irradiation at optimum conditions. The degradation efficiency of textile effluent was found to be 24.65% for PRCS and 53.99% for charcoal at a contact time of 120 min, because the increased surface area of the charcoal decays the maximum percentage of organic pollutant than the prepared adsorbents. The comparison of degradation capacities of the textile effluent onto PRCS and charcoal was given in the Table. The UV- absorbance spectrums was also shown in the Figs.11 and 12.
 |
Figure 11: UV spectra of textile effluent onto PRCS. |
 |
Figure 12: UV spectra of textile effluent onto Charcoal |
Table 3: Degradation capacities of the textile effluent onto PRCS and charcoal
|
Adsorbent |
Stimulated waste water |
Effluent |
||
|
Time(min) |
Degradation |
Time(min) |
Degradation |
|
|
PCRS |
120 |
82.79 % |
120 |
24.65 % |
|
Charcoal |
– |
– |
120 |
53.99 % |
Column studies for the Textile effluent onto PRCS
For adsorption purpose mini-columns was used, each column was packed with a layer of cotton, above the cotton 1 g PRCS was filled with bed height around 20mm. Flow rate of dye was 2ml per minute throughout the experiments. The concentration of the effluent was detected by measuring their absorbance using UV spectrophotometer. The contact time with a given part of the adsorbent was limited, a true equilibrium was never reached, which was shown in Fig.13.
 |
Figure 13: Column studies for the Textile effluent onto PRCS |
Kinetic Studies
The photocatalytic degradation of methyl red dye by PRCS adsorbent is explained by the kinetic studies. The kinetics was analysed by using pseudo I order and pseudo II order models. The correlation regression coefficient R2 and maximum adsorption capacity qe of the adsorption of MR dye on PRCS are 0.9939 and 18.93(mg/g min). This is indicated that the adsorption kinetics on MR dye is fitted for pseudo second order kinetics. The kinetic data are given in the Table. 4. Kinetic models were detected by using the following formulas which is given below.
Pseudo first order equation
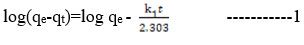
Pseudo second order equation

Table 4: Kinetic variables for the Degradation of MR Dye onto PRCS
|
Kinetic models |
Parameters |
Data |
|
Pseudo- first order |
k1 |
0.043 min-1 |
|
qe |
11.36 mg/g |
|
|
R2 |
0.8777 |
|
|
Pseudo-second order |
k2 |
0.0013 g/mg min |
|
H |
0.429 |
|
|
qe |
18.93 cal(mg/g) |
|
|
R2 |
0.9919 |
Isotherm studies
In this present studies, Langmuir and Freundlich isotherm models were performed. The experimental equilibrium isotherm data were obtained by changing the concentration of MR with 0.25 g/50 mL is shown in Table 5. Equations for these models were illustrated in equations 3 and 4. The results revealed that the Langmuir Isotherm model is best fit for the equilibrium data than Freundlich Isotherm model.
Langmuir

Freundlich
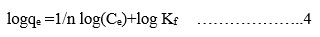
Table 5: Isotherm Parameters for the Adsorption of Mr
|
Isotherm Models |
Pparameters |
Data |
|
Langmuir Isotherm |
Qm |
8.1234 Mg/G |
|
B |
2.25 L/Mg |
|
|
R2 |
0.9951 |
|
|
Freundlich Isotherm |
N |
2.96 |
|
Kf |
3.033 Mg/G |
|
|
R2 |
0.9291 |
Mechanism For Dye Degradation
The activated Ricinus Communis stem is used as a photo catalyst is the primary reason for the degradation of MR dye, when it is exposed to direct solar light irradiation it gives electron hole pairs. The dye degradation is induced by the formed electron hole pairs. The initial dye concentration is the cause for the photo degradation of dyes. Even though it is dye degradation initially adsorption of the dye on the studied adsorbents occurs followed by photo degradation process. At the initial stage more vacant sites are available on the surface on the adsorbent for adsorbing dye molecule when the concentration of the dye is low, but at the later stages the availability of the vacant sites are less on the surface of the adsorbent for adsorbing dye molecule when the concentration of the dye is high. Because, the vacant sites are filled by the dye molecules therefore the rate of dye degradation decreases.
Mechanism of photo degradation occurs in two steps, i.e., Dye adsorption by adsorbent followed by degradation under direct sunlight takes place by the following equation
Adsorbent+ hν → Adsorbent (e–+h+)
O2+ e– →O2∙-
O2∙- + e– + 2H+ → 2OH ∙
Oxygen plays a vital role in the degradation of dyes. A sunlight, oxygen and hydroxyl ion shows considerable effects on the degradation. If reduction of any one of the species will decrease the degradation capacity of the dye. The formed hydroxyl radical species react with anything to form hydrogen peroxide, which is a considerable active species in photo degradation process.
2OH ∙ →H2O2
The formed hydrogen peroxide cleaved to form OH ∙
H2O2 + hν → 2OH
Degradation of dye by OH ∙ radical
OH ∙ ads + dye → oxidation of the dye
Conclusion
In this paper the degradation ability of PRCS is studied. Chemical activation of Ricinus communis stem with phosphoric acid gives high yield and high surface area of activated carbon. The physiochemical properties are investigated. The surface analysis of activated carbon was experimented by SEM, EDX, XRD. The effect of pH, Contact time, Adsorbent dose, initial concentration of dye on Degradation of methyl red were analysed. The maximum degradation efficiency of MR dye on PRCS is 82.79%. The degradation of textile effluent, stimulated waste water onto PRCS were analysed by using sunlight irradiation and column studies. The degradation efficiency of textile effluent was found to be 24.65% for PRCS. The degradation efficiency was confirmed by UV spectra. The kinetic studies were also investigated by varying concentration with contact time. The single component equilibrium characteristics of degradation of MR dye by two different isotherm models were investigated. The experimental equilibrium isotherm data were good agreement with calculated values. This paper concluded using activated carbon of Ricinus communis stem is an inexpensive adsorbent for the removal of methyl red dye from waste water.
Conflict of Interest
There is no conflict of interest
Funding Sources
There is no funding source.
References
- K. A. Adegoke And O. S. Bello, Water Resour. Ind., 12, 8 (2015); Https://Doi.Org/10.1016/J.Wri.2015.09.002
CrossRef - A.H. Jawad, S.S.A. Norrahma, B.H. Hameed And K. Ismail, Int. J. Biol. Macromol.,135, 569(2019); Doi: 10.1016/J.Ijbiomac.2019.05.127
- A.Oussalah, A. Boukerroui, B.Aichour And B. Djellouli., Int.J. Biol. Macromol ., 124, 854(2019); Https://Doi.Org/10.1016/ J.Ijbiomac. 2018.11.197
CrossRef - Z.Tahira, A.Aslam, M. Abbas, M. Mehboob, S. Ali And A. Asghar., Int. J. Biol. Macromol.,136, 1209 (2019).; Https://Doi.Org/10.1016 /J.Ijbiomac. 2019.06.173
- W.C.Lim , C.Srinivasakannan And A.A.Shoaibi, J.Clean.Product ., 102, 501 (2015);Https://Doi.Org/10.1016/ J.Jclepro.2015.04.100
CrossRef - C.Namasivayam , N.Muniasamy , K. Gayathri , M. Rani And K. Ranganathan , Bioresource Technol., 57, 37 (1996); Https://Doi.Org/10.1016/0960-8524(96)00044-2
- T.B. Robinson, T, B. Chandran And P. Nigam, Water Res ., 36, 2824 (2002); Doi: 10.1016/S0043-1354(01)00521-8
CrossRef - V.Garg, R.Gupta., A.B. Yadav And R. Kumar, Bioresour.Technol. ., 89, 121 (2003); Doi: 10.1016/S0960-8524(03)00058-0
CrossRef - I.Haq , H.N.Bhatti And M. Asgher., Canad.J.Chem.Eng..,89, 593 (2011); Https://Doi.Org/10.1002/Cjce.20436
CrossRef - M.A. Ahmad, N. Ahmad, And Bello, O.S. Model. Earth Syst. Environ. 3, 25 (2017); Https://Doi.Org/10.1007/S40808-017-0269-0
CrossRef - M.A.Ahmad , N.S.Afandi And O.S.Bello., Appl.Water Sci ., 7, 717 (2017), Doi:10.1007/S13201-015-0284-0.
CrossRef - T.Santhi, S.Manonmani And T.Smitha., Journal Of Hazardous Materials., 179, 178(2010); Https://Doi.Org/10.1016/ J.Jhazmat. 2010.02.076
CrossRef - Vineet Kumar, Luiz Fernando Romanholo Ferreira, Madan Sonkar, Joginder Singh, Journal Of Environmental Technology & Innovation Volume 22, 2021, 101382, Https://Doi.Org/10.1016/J.Eti.2021.101382.
CrossRef - S. Madhavakrishnan, K. Manickavasagam, R. Vasanthakumar, K. Rasappan, R. Mohanraj And S. Pattabhi, Journal Of Chemistry., 6, (2009); Https://Doi.Org/10.1155/2009/764197.
CrossRef - H.Y. Zhu, R. Jiang, J.H. Jiang, L. Xiao And G.M. Zeng, Appl. Surf. Sci., 258,1337 (2011); Doi10.1016/J.Apsusc.2011.09.045
CrossRef - S. Archin, S. H. Sharifi And G. Asadpour, J.Cleaner Production., 239, 118 (2019); Doi: 10.1016/J.Jclepro.2019.118136
CrossRef - R.K. Liew, E.Azwar, P.Y.Yek, X.Y. Lim. And C.K. Cheng, Bioresour. Technol , 266, 1 (2018); oi: 10.1016/J.Biortech.2018.06.051
CrossRef - E.L.Ahmed , O. Abdelwahab, A. E. Sikaily And A. Khaled., Journal Of Hazardous Materials., 161, 102 (2009); Doi: 10.1016/ J.Jhazmat. 2008.03.060.
CrossRef - Furlan F.R, De Melo De Silva L.G, Morgado A.F, Do Souza A.A.U, De Souza Smagu (2010) Resour Conserv Recycl 54:283–290
- M.Makeswari. And T.Santhi., J.Water Resource And Protection ., 5,2 (2013); Doi: 10.4236/Jwarp.2013.52023
CrossRef - M..Makeswari. And P.Saraswathi, Sn Applied Sciences., 2,336 (2020); Doi:10.1007/S42452-020-1980-4
CrossRef - M.Makeswari. And T,Santhi, Journal Of Chemistry., 12 (2013);. Https://Doi.Org/10.1155/2013/314790
CrossRef - Mohd Muslim, Arif Ali, Saima Kamaal, Musheer Ahmad, Mohammad Jane Alam, Qazi Inamur Rahman, M. Shahid, Journal Of Molecular Liquids, 2021, 117951, Https://Doi.Org/10.1016/J.Molliq.2021.117951.
CrossRef - B.A. Khalid Siraj And N.Meka, Green Chemistry Letters And Reviews ., 8,1 (2015); Doi:10.1016/J.Jenvman.2009.12.016
CrossRef - R. Chikri,N. Elhadiri, M. Benchanaa, And Y. El Maguana, Journal Of Chemistry., ( 2020); Https://Doi.Org/10.1155/2020/8813420
CrossRef - A. Amina , A. Badie, S.Girgis, And N,.A. Fathy, Dyes And Pigments., 76,282 (2008); .Https://Doi.Org/10.1016/J.Dyepig.2006.08.039
CrossRef
Accepted on: 25-Jan-2022
Second Review by: Dr. Luma Alnakash
Final Approval by: Dr. Bal krishan

















