Simultaneous Detection of Pb(II) and Cd(II) Ions in Noodle Soup Samples using Square Wave Anodic Stripping Voltammetry
Prawit Nuengmatcha*1 , Benjawan Ninwong1and Saksit Chanthai2
, Benjawan Ninwong1and Saksit Chanthai2
1Nanomaterials Chemistry Research Unit, Department of Chemistry, Faculty of Science and Technology, Nakhon Si Thammarat Rajabhat University, Nakhon Si Thammarat 80280, Thailand.
2Materials Chemistry Research Center, Department of Chemistry and Center of Excellence for Innovation in Chemistry, Faculty of Science, Khon Kaen University, Khon Kaen 40002, Thailand.
Corresponding Author E-mail: pnuengmatcha@gmail.com
DOI : http://dx.doi.org/10.13005/ojc/350243
Article Received on : 08-05-2018
Article Accepted on : 05-04-2019
Article Published : 28 Apr 2019
Noodles are a favorite food in many cultures. There are many cases that noodle soups were contaminated with heavy metal like Pb(II) and Cd(II) which released from their container or boiling pot. In this work, the highly selective method has been established for simultaneous detection of Pb(II) and Cd(II) ions using bismuth film electrode by applying square wave anodic stripping voltammetry or SWASV technique. Bi(III) ion was used to enhance analytical signal by in situ plating solutions on glassy carbon support. The electrochemical analysis is based on simultaneous preconcentration /reduction of both ions at -1.1 V (versus Ag/AgCl) in 0.5 molL-1 HCl during 240 s, followed by subsequent chemical stripping of square-wave detection. The linear calibration curve was exhibited in the ranged of 0.02-1.0 mgL-1. The value of limits of detection (LOD) for Pb(II) and Cd(II) were attained at 0.007 mgL-1 and 0.004 mgL-1. The developed method was successfully used to simultaneous determine Pb(II) and Cd(II) ions in noodle soup samples from noodle shops in Nakhon Si Thammarat Province, Thailand. The recoveries were in the range 83-109%. The present method provides highly selective detection for determination of both Pb(II) and Cd(II) ions.
KEYWORDS:Cadmium(II); Lead(II), Noodle Soup Samples; Voltammetry
Download this article as:| Copy the following to cite this article: Nuengmatcha P, Ninwong B, Chanthai S. Simultaneous Detection of Pb(II) and Cd(II) Ions in Noodle Soup Samples using Square Wave Anodic Stripping Voltammetry. Orient J Chem 2019;35(2). |
| Copy the following to cite this URL: Nuengmatcha P, Ninwong B, Chanthai S. Simultaneous Detection of Pb(II) and Cd(II) Ions in Noodle Soup Samples using Square Wave Anodic Stripping Voltammetry. Orient J Chem 2019;35(2). Available from: https://bit.ly/2ILmofx |
Introduction
Noodles are a staple food in many countries. They are cooked in boiling water, sometimes with cooking oil or salt added. When their container contact with food, the impurities may transfer into the food. Particularly, Pb(II)) and Cd(II) are known to be high toxic metals to not only aquatic life but also human.1 They could accumulate via food chain which cause harm to human health. Hence, the development technique for determination of both ions in noodle soups samples is very significance for not only the human safety but also pollution control.2 Recently, several analytical techniques have been used to determine the quantity of Pb(II)and Cd(II) ions in various samples such as spectrometry techniques (AAS, ICP, UV-Vis), chromatography technique (HPLC) and liquid ion exchange.3-11 However, these techniques are rather limited in practical including time-consuming including tedious sample preparation and specific operating skills.
Among these, square wave anodic stripping voltammetry or SWASV technique is one of the most widely used due to its unique properties including low limit of detection, high sensitivity, low cost and wide dynamic range. Therefore, this technique was applied to determine various target analysts such as cadmium lead (Pb(II)), (Cd(II)), arsenic
(As(III)), mercury (Hg(II)), gallium (Ga(III)), zinc (Zn(II)),indium (In(III)), copper (Cu(II)), oleuropein, tryptamine, and levofloxacin.12-23 However, these works show the suitable techniques for the detection of only single target analyst. Therefore, simultaneous detection by SWASV is in high demands and remains a challenge for determination of toxic metal ions quantity in real samples.
This work proposes to establish a highly selective technique for simultaneous detection of Pb(II) and Cd(II) which are toxic ions at trace levels by using SWASV. The optimum conditions including deposition potential, supporting electrolyte, plating concentration, frequency, step potential, pulse amplitude and time were investigated. The established technique was successfully used to determine the trace quantity of Pb(II) and Cd(II) ions in noodle soup samples.
Materials and Methods
Chemicals and Apparatus
All chemical reagents were used without further purification and prepared using distillation water. HCl, CH3COOH and HNO3 were purchased from Merck, Germany. And CH3COONa, Na2HPO4×7H₂O. and NaH2PO4×H2O were purchased from Fluka Chemika, Switzerland. The working standard of Pb(II) and Cd(II) were prepared by diluting from 1,000 mg L–1 of AAS stocked standard solution. After that, the samples were diluted with 0.5 mol L–1 HCl which worked as a supporting electrolyte. A 1 mg L–1 of Bi(III) ions in 0.5 mol L–1 HCl was prepared by the dilution of Bi(III) 1000 mg L–1. CH3COONa, CH3COOH and 3 mol L–1 HCl were applied to prepare the 0.1 mol L–1 acetate buffer of the preferred pH. Na2HPO4×7H₂O. and NaH2PO4×H2O were used to study of pH effect. The AAS solution standard of Al(III), Cr(III), Zn(II), Cu(II), Fe(III) and Hg(II) was used for interference study. All laboratory glasswares were soaked in a 10% (v/v) HNO3 solution to prevent metal contamination. Anodic stripping voltammetry was performed by using an Autolab Potentiostat (Autolab 101, Netherlands). The bath system consisted of glassy carbon electrode, an Ag/AgCl and a platinum were the working electrode, reference electrode and counter electrode, respectively. The system was set for batch analysis with in situ bismuth plating solution.
Procedure
To study the simuntaneous detection of Pb(II) and Cd(II) ions, the ASV was applied by in situ 1 mg L–1 Bi(III) plating which was electrodeposited on the surface of electrode at -1.1 V vs. Ag/AgCl. The working electrode were deposited with these ions under the optimum potential for 240 min. After that, all samples were recorded their voltammogram in the range of -1.1 to 0.4 V using the scanning a potential technique via the square-wave waveform under suitable conditons including 100 Hz, 5 mV and 30 mV for frequency, step potential and pulse amplitude, respectively. In addition, to clean the electrode, 1 mol L-1 HCl was applied to remove the residual metal ions at +1 V for 60 min. The procedure of this method was optimized for supporting electrolyte, the concentration of plating solution, frequency, step potential, pulse amplitude, deposition potential and time.
Application
The Bi(III) plating electrode was applied to determine the concentration of Pb(II) and Cd(II) ions in noodle soup samples. All samples were collected from varous noodle shops in Nakhon Si Thammarat Province, Thailand. Briefly, 10 mL of 0.1 mol L–1 HNO3 and 15 mL of noodle soup sample were mixed. After that, the mixtures were heated to evaporate an excess water until 2.5 mL of the sample remained.24 Finally, their volume and pH were adjusted by using buffer solution. To validate the proposed method, ICP-OES technique was used to determine the quantity of Pb(II) and Cd(II) ions. In addition, the accuracy was tested by spiked standard solution at 0.1, 0.5 and 0.7 mg L–1.
Results and Discussion
Effect of Bi(III) Concentration
Bi(III) was used to modify working electrode for an enhancement of the current of ASV25,26. The Bi film was depended on Bi(III) concentration, which affected the voltammogram. So, the effect of Bi(III) concentration plating was studied on ASV of Pb(II) and Cd(II) in the range of 0.25 to 2.0 mgL–1. From Fig.1a, the currents of Pb(II) and Cd(II) drastically increased with the increasing of Bi(III) concentration up to 1.0 mgL–1 and then clearly decreased at concentration of Bi(III) higher than 1 mgL–1. Bi(III) ion helped induction the target metal ions to accumulate on glassy carbon electrode as used for working electrode. The high concentration of Bi(III) ion decreased the signal current caused by limitation of the area on working electrode. Therefore, the suitable Bi(III) concentration of 1 mgL–1 was chosen indicating the highest ASV peak current.
Effect of Supporting Electrolyte
The effect of electrolyte influents on metal ions that has different electrochemical detection in each electrolyte solution. The widely used electrolytes such as HCl, acetate buffer solution and phosphate buffer solution were subject to study by ASV for Pb(II) and Cd(II) detection. The results show that Pb(II) and Cd(II) give the best electrochemical responses in HCl with good stripping peak. Moreover, the influence of HCl concentration for Pb(II) and Cd(II) detection was also considered in the range 0.01 to 1.0 mol L–1 (Fig. 1b). The peak current increased at 0.5 mol L–1 and decreased when higher concentrations, because continuous increases in the concentration of HCl could produce bubble in the solutions resulted in reduction of working electrode area. So, the maximum of peak current reached at 0.5 mol L–1 which was suitable for supporting electrolyte.
Deposition Potential Effect
The deposition potential influence on the obtained stripping peak current of two metal ions was performed in the range of potential from -0.8 to-.2 V vs. Ag/AgCl. The results were shown in Fig. 1c. The negative changes of deposition potential could improve the reduction of Pb(II) and Cd(II) on the working electrode surface and increased the stripping peak current. However, the deposition potential which found to be maximum current was -1.2 V vs. Ag/AgCl. In contrast, some other metal ions may be decreased at these potentials. Therefore, the deposition potential at -1.1 V was applied to complete both high sensitivity and better response for electrochemical detection.27
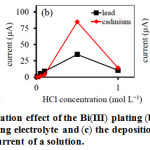 |
Figure 1: (a) the concentration effect of the Bi(III) plating (b) effect of the concentration of supporting electrolyte and (c) the deposition potential effect on the ASV peak current of a solution. |
Deposition Time Effect
The deposition time is vary importance parameter for ASV technique. Figure 2 showed the deposition time effect on the currents of metal ions. From the result, it was found that the deposition time greatly increased in the range of 60 s to 300 s. In addition, the stripping peak currents decreased after the deposition time higher than 300 s was applied, which was probably due to the saturation of electrode surface loading, so the excess deposition of Bi(III) film on the working electrode was similar to the Bi(III) concentration effect. However, the highest peak current was occurred at 300 s that is long time for deposition of metal ions. Thus, the accumulation time of 240 s was chosen for the reduction time of detection and cleaning electrode.
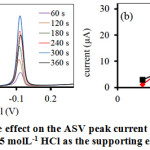 |
Figure 2: The deposition time effect on the ASV peak current of a solution of 0.5 mgL-1 Pb(II) and Cd(II) in 0.5 molL-1 HCl as the supporting electrolyte. |
Voltammetric Detection Effect
To determine the quantity of Pb(II) and Cd(II) by using SWASV technique, the key parameters including step potential, influent of square-wave frequency and amplitude were also investigated. The stripping currents of two metal ions increased with the increasing of the square-wave frequency which were related for high scan rate and then it decreased over 100 Hz (Fig. 3a). As similar to amplitude increased up to 30 mV as shown in Fig. 3b. The increasing of the amplitude may be due to a raise in the peak currents for both two metal ions, since the cathodic and anodic currents were larger difference. Hence, the optimum frequency and amplitude were chosen as 100 Hz and 30 mV, respectively. An increase in the step potential, signal of Pb(II) increased but the peak current of Cd(II) decreased at higher than 7.5 mV (Fig. 3c). So, the optimum step potential for square-wave voltammetry was at 5 mV.
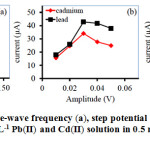 |
Figure 3: The effect of square-wave frequency (a), step potential (b) and amplitude (c) on the ASV peak current of 0.5 mgL-1 Pb(II) and Cd(II) solution in 0.5 molL-1 HCl as the supporting electrolyte. |
Effect of Interference
To study the selectivity of the developed method. The effect of other ions including Zn(II), Cr(III), Cu(II), Al(III), Fe(III) and Hg(II) in the solution sample as interference ions on the ASV current for Pb(II) and Cd(II) ions detection were considered. The effect of these interferences was displayed as the preferred analyte to interference concentration ratio. The responses of Pb(II) and Cd(II) ions solution were compared with the analytical responses of the interfering ions addition at 1:1, 1:3 and 1:5 ratios. The result from Fig. 4. showed that all metal ions of interferences had no significant affecting on the analytical signals of Pb(II) and Cd(II) due to the negative or positive change of sample response less than 10-15% which was compared to the Pb(II) or Cd(II) ions response alone.28
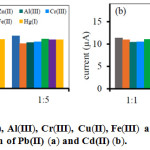 |
Figure 4: The effect of Zn(II), Al(III), Cr(III), Cu(II), Fe(III) and Hg(II) ions on the ASV current for the determination of Pb(II) (a) and Cd(II) (b). |
Analytical Performance
Under the suitable conditions (Table 1), Fig. 5 showed the calibration curves of Pb(II) and Cd(II). The linear range was found to be 0.02-10 mgL–1, the analytical response was performed at low concentration in the range of 0.02-1.0 mgL–1, R2 = 0.9960 and R2 = 0.9968 for Pb(II) and Cd(II), respectively. The limits of detection (LOD = 3SD) were found at the concentrations of 0.007 mg L–1 for Pb(II) and 0.004 mgL–1 for Cd(II). The limit of quantification (LOD = 10SD) for two metal ions was found to 0.02 mgL–1. After 7 measurements, the relative standard deviations (%RSD) for different concentrations not over than 15% and non-significant for inter-day analysis which indicated that the proposed method has high precision for Pb(II) and Cd(II) determination.
Table 1: Optimum conditions for the determination of Pb(II) and Cd(II) by ASV.
| Parameter |
Range examined |
Optimized condition |
| Electrolyte concentration (mol L–1) |
0.01 to 1.0 |
0.5 |
| Conc. of Bi plating solution (mg L–1) |
0.25 to 2.0 |
1.0 |
| Deposition potential (V) |
-0.8 to -1.2 |
-1.1 |
| Deposition time (s) |
60 to 360 |
240 |
| Frequency (Hz) |
5 to 125 |
100 |
| Step potential (mV) |
1 to 10 |
5 |
| Amplitude (mV) |
10 to 50 |
30 |
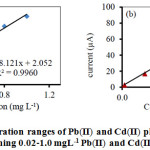 |
Figure 5: The linear concentration ranges of Pb(II) and Cd(II) plotting the ASV peak currents of a solution containing 0.02 1.0 mgL–1 Pb(II) and Cd(II) ions in 0.5 molL-1 HCl.Click here to view figure |
Conditions: Bi(III) concentration plating: 1 mgL-1; frequency: 100 Hz, pulse amplitude: 0.030 V, step potential: 0.005 V and deposition potential: -1.1 V.
Application in Noodle Soup Samples
The developed procedure was applied for determination of Pb(II) and Cd(II) in noodle soup samples which obtained from noodle shops in Nakhon Si Thammarat Province, Thailand. All samples were divided into 3 portions. After that, various concentration of standard solution were spiked into the samples including 0.1, 0.3 and 0.7 mgL–1. The obtained results were summarized in Table 2. The recoveries of two metal ions were acceptable which demonstrated in a range of 83 to 109%. In addition, from the comparison of analyze performance between the developed method and ICP-OES method, it was found to be no significant difference. Therefore, it is shown that the developed method can be successfully implied for the Pb(II) and Cd(II) determination in noodle soup samples.
Table 2: The recoveries of Pb(II) and Cd(II) determination in noodle soup samples by the developed method.
|
Sample |
Spiked level |
Found (mg L-1) |
Recovery (%) |
|||
|
(mg L-1) |
Pb(II) |
Cd(II) |
Pb(II) |
Cd(II) |
||
|
Sample 1 |
0.00 |
N.D. |
N.D. |
|||
|
0.10 |
0.099 ± 0.36 |
0.108 ± 0.25 |
99 |
108 |
||
|
0.50 |
0.547 ± 0.45 |
0.481 ± 0.36 |
109 |
96 |
||
|
0.70 |
0.623 ± 0.55 |
0.696 ± 1.57 |
89 |
99 |
||
|
Sample 2 |
0.00 |
N.D. |
N.D. |
|||
|
0.10 |
0.098 ± 0.35 |
0.105 ± 1.21 |
98 |
105 |
||
|
0.50 |
0.437 ± 0.26 |
0.416 ± 1.16 |
87 |
83 |
||
|
0.70 |
0.725 ± 1.20 |
0.717 ± 0.85 |
103 |
102 |
||
|
Sample 3 |
0.00 |
N.D. |
N.D. |
|||
|
0.10 |
0.091 ± 0.28 |
0.105 ± 0.67 |
91 |
105 |
||
|
0.50 |
0.549 ± 1.30 |
0.446 ± 0.26 |
109 |
89 |
||
|
0.70 |
0.701 ± 0.33 |
0.699 ± 0.75 |
100 |
99 |
||
N.D. = not detected
Conclusion
In this work, the selective method has been developed for the simultaneous determination of Pb(II) and Cd(II) in noodle soup samples by using square-wave anodic stripping voltammetry. A good analytical performance is present including highly selectivity, easy to operate and cost-efficient for trace metal ions over traditional method. The practical analysis was successfully applied to low concentration level of metal ions in noodle soup samples. In addition, the proposed method can also serve well for complex matrix samples.
Acknowledgements
This research was financially supported by Nanomaterials Chemistry Research Unit, Department of Chemistry, Faculty of Science and Technology, Nakhon Si Thammarat Rajabhat University.
References
- Zhao, S.L.; Chen, F.S.; Zhang, J.; Ren, S.B.; Liang, H.D.; Li, S.S. J. Ind. Eng. Chem. 2015, 27, 362-367.
- Guo, X.; He, M.; Chen, B.; Hu, B. Talanta. 2012, 94, 70-76.
- Aleluia, A.C.M.; Santana, F.A.D.; Brandao, G.C.; Ferreira, S.L.C. Microchem. J. 2017, 130, 157-161.
- Malik, A.A.; Mohamed, H.; Hassan, M.; Awad, A.M. Orient. J. Chem. 2017, 33, . 2263-2270.
- Zhao, X.; Wei, J.; Shu, X.; Kong, W.; Yang, M. Chemosphere. 2016, 164, 430-435.
- Li, W.; Wang, C.; Gao, B.; Wang, Y.; Jin, X.; Zhang, L.; Sakyi, P.A. Microchem. J. 2016, 127, 237-246.
- Guo, X.; He, M.; Chen, B.; Hu, B. Talanta. 2012, 94, 70-76.
- Hu, Q.; Yang, G.; Yin, J.; Yao, Y. Talanta. 2002, 57, 751-756.
- Barałkiewicz, D.; Kózka, M.; Piechalak, A.; Tomaszewska, B.; Sobczak, P. Talanta, 2009, 79, 493-498.
- Wen, X.; Deng, Q.; Guo, J.; Yang, S. Spectrochim. Acta, Part A. 2011, 79, 508-512.
- Shawket, K.J.; Maha, A.H. Orient. J. Chem. 2017, 33, 2452-2458.
- Deswati; Izzati, R.; Hamzar, S., Rahmiana, Z.; Admin, A. Orient. J. Chem. 2016, 32, 1493-1502.
- Kamyabi, M.A.; Aghaei, A. Electrochim. Acta. 2016, 206, 192-198.
- Illuminati, S.; Annibaldi, A.; Truzzi, C.; Libani, G.; Mantini, C.; Scarponi, G. J. Electroanal. Chem. 2015, 755, 182-196.
- Illuminati, S.; Annibaldi, A.; Truzzi, C.; Scarponi, G. Mar. Pollut. Bull. 2016, 111, 476-482.
- Laffont, L.; Hezard, T.; Gros, P.; Heimbürger, L.E.; Sonke, J.E.; Behra, P.; Evrard, D. Talanta. 2015, 141, 26-32.
- Kamat, J.V.; Guin, S.K.; Pillai, J.S.; Aggarwal, S.K. Talanta. 2011, 86, 256-265.
- Deswati; Hamzar, S., Izzati, R.; Hilfi, P. Orient. J. Chem. 2017, 33, 2060-2070.
- Charalambous, A.; Economou, A. Anal. Chim. Acta. 2005, 547, 53-58.
- Harmoudi, H.E.; Achak, M.; Farahi, A.; Lahrich, S.; Gaini, L.E.; Abdennouri, M; Bouzidi, A.; Bakasse, M.; Mhammedi, M.A.E. Talanta. 2013, 115, 172-177.
- Cittan, M.; Koçak, S.; Çelik, A.; Dost, K. Talanta. 2016, 159, 148-154.
- Costa, D.J.E.; Martínez, A.M.; Ribeiro, W.F.; Bichinho, K.M.; Nezio, M.S.D.; Pistonesi, M.F.; Araujo, M.C.U. Talanta. 2016, 154, 134-140.
- Radi, A.; El-Sherif, Z. Talanta. 2002, 58, 319-324.
- Ratnarathorn, N.; Chailapakul, O.; Henry, C.S.; Dungchai, W. Talanta. 2012, 99, 552-557.
- Wang, J.; Lu, J.; Hocevar, S.B.; Ogorevc, B. Electroanalysis. 2001, 13, 13-16.
- Lee, G.J.; Lee, H.M.; Rhee, C.K. Electrochem. Commun. 2007, 9, 2514-2518.
- Li, Y.; Liu, X.; Zeng, X.; Liu, Y.; Liu, X.; Wei, W.; Luo, S. Sens. Actuators, B. 2009, 139, 604-610.
- Tarley, C.R.T.; Santos, V.S.; Baêta, B.E.L.; Pereira, A.C.; Kubota, L.T. J. Hazard. Mater. 2009, 169, 256-262.

This work is licensed under a Creative Commons Attribution 4.0 International License.









