Phytoremediation of Heavy Metals Spiked Soil by Polyscias Fruticose
Naseer Inuwa Durumin Iya*1,2 , Zaini Bin Assim1, Isa Bin Ipor1, Saminu Murtala Yakasai2, Shina Ismail Sadiq2 and Binta Hadi Jume3
, Zaini Bin Assim1, Isa Bin Ipor1, Saminu Murtala Yakasai2, Shina Ismail Sadiq2 and Binta Hadi Jume3
1Faculty of Resource Science and Technology, Universiti Malaysia, 94300 Kota Samarahan, Sarawak, Malaysia.
2Department of Chemistry, Federal University Dutse, Ibrahim Aliyu Bye-Pass, 7156 – Dutse, Jigawa State, Nigeria.
3Department of Chemistry, Preparatory School, Aljoup University, Sakaka, 72388, Aljoup Region, KSA.
Corresponding Author E-mail: nasduruminiya@yahoo.com
DOI : http://dx.doi.org/10.13005/ojc/350135
Article Received on : 24-11-2018
Article Accepted on : 10-01-2019
Article Published : 08 Feb 2019
This study was to assess the phytoremediation potential of Polyscias fruticose by removing heavy metals from spiked soil. P. fruticose cuttings were transplanted then grown on 2.00 kg soil spiked with several heavy metals in polyethylene bags. The experiment was conducted for 300 days and heavy metals concentration differences in plant and soil over the growth periods was determined. Appreciable concentrations of heavy metals in plant organs were obtained. The indices used to show the ease of heavy metals uptake and translocation indicated that Co, Cr, Mn, Ni and Pb displayed the greatest ease of absorption while Zn, Fe and Cu were absorbed but not translocated to the shoot. The results obtained shows that this study pioneered the use of P. fruticose in the phytoremediation of several heavy metals spiked soil at a greenhouse level.
KEYWORDS:Bio-Concentration Factor; Heavy Metal; Phytoremediation; Phytoextraction; Polyscias Fruticose; Spike
Download this article as:| Copy the following to cite this article: Iya N. I. D, Assim Z. B, Ipor I. B, Yakasai S. M, Sadiq S. I, Jume B. H. Phytoremediation of Heavy Metals Spiked Soil by Polyscias Fruticose. Orient J Chem 2019;35(1). |
| Copy the following to cite this URL: Iya N. I. D, Assim Z. B, Ipor I. B, Yakasai S. M, Sadiq S. I, Jume B. H. Phytoremediation of Heavy Metals Spiked Soil by Polyscias Fruticose. Orient J Chem 2019;35(1). Available from: https://bit.ly/2SkMyL3 |
Introduction
The world industrial activities, such as electroplating, fertilizer, municipal solid waste, smelting, gas exhaust, pesticides, fuel production, iron ores, energy production and mining have a large contribution in the release of significant amounts of toxic compounds into the biosphere, among which are trace heavy metals.1 Human activities, urbanization and industrial development discharge harmful wastes into the environment, such as soil, water and air. These harmful wastes are classified as contaminants, and their outcomes are harmful and toxic to the soil, water, air, human, animals, plants and microorganism. Common harmful heavy metals are Al, Cr, Cd, Cu, Pb, Mn, Zn, Hg and some metalloids like Sb and As are also harmful.2 Heavy metals are dangerous to terrestrial and aquatic ecological community, and affect the health of human and animal.3 They get in contact with human and animal body system through food chain, water, atmospheric air and also direct or indirect contact with the skin.2 Their major health problem related to consumption or coming in contact with heavy metals are cardiovascular disease, prolonged anaemia, cognitive disability,4 cancer problem, kidneys diseases,5 problem of nervous system and brain damage, skin infection, bones and teeth.2 Reducing harmful heavy metals from the environment is necessary and very important in order to prevent the environment from the harmful effects of heavy metals and also save it for future uses. Many physical, chemical and biological techniques have been applied for the removal and reduction of heavy metals, which is considered to be cost effective and more complex.1 The use of plant to reduce or remove harmful contaminants from polluted environment is defined as biological remediation.6 Biological remediation is considered to be the most efficient method of heavy metal reduction or removal because it is a natural process, environmentally friendly, less expensive, no machine application and publically accepted. The techniques include land forming, bioremediation, bioventing, bioleaching, bioreactors, bio-remediation composting, phytoremediation, bio-augmentation and bio-stimulation. Among these techniques, bio-remediation and phytoremediation are the most important methods.6 These techniques have more advantages over physical and chemical techniques because they preserve natural soil properties, less expensive and depend on solar energy.6 Therefore, the aim and objectives of this study were (a) to assess the ability of P. fruticose to survive and grow on a soil spiked with several heavy metals (b) and to evaluate the uptake and translocation of heavy metals to aboveground tissues. The novelty of the present study is the potential of P. fruticose to grow in a heavy metals contaminated soil, extract, accumulate and translocate heavy metals to aboveground tissues in a normal plant growth condition at the greenhouse. The outcome of the experiment shows that P. fruticose was effective and efficient for the phytoremediation of soil spiked with several heavy metals.
Materials and Methods
The study was performed at the greenhouse of Faculty of Resource Science and Technology, Universiti Malaysia Sarawak, (N 01o 33’ 03.6’’ E 110o 45’ 56.5’’) from 17th April 2017 to 26th March, 2018. The first harvest of the first set of P. fruticose after 2 months took place on 20th Jun, 2017.
Apparatus and Instrumentations used
Perkin Elmer Atomic Absorption Spectrometer (AAS) model Optima 8300 series, Stuart/FX105-27 series muffle furnace, Memmert oven model XMM-UNB 200, stainless steel mesh BS 410-1:2000 model, Favorit HS 0707V2 series hot plate, polyethylene bag, weighing balance AND model FX-300i series 15621105 and scoop.
Soil and Plant Samples
In this study Polyscias fruticose (Ming Aralia) plant was selected for its ability to produce good root system and more biomass. It is from the family of Araliaceae, Polyscias is the genus and fruticose is its species. Fresh young plant samples of P. fruticose were collected within the Universiti campus and about 5.0 cm cuttings were made and kept for 3 h to dry. About 2.5 cm from the bottom of the cutting were scraped (one side) with a sterilized surgical blade to enable the growth of roots. These cuttings were used for pot experiment. The top soil was collected from Kota Samarahan, Kuching, it was allowed to dry and larger particles were removed and homogenized.
Pot Experiment
Greenhouse experiment was conducted according to the procedure described in8 with minor modification. Poly bags were filled with 2.0 kg soil spiked with a 50 mg/kg solution containing a mixture of several heavy metals at a greenhouse. Six (6) sets of cuttings each contain five (n=5) replicates which include control were set as required in a growth archaic. The greenhouse growth conditions were 9 hours dark at 19°C and 24°C for 15 hours daylight. The plants were watered every other day and were netted. Plants were gathered from poly bags and washed with distilled water after every 2 months for the period of 10 months. The plants were separated in to root, stem and leaf then dried in an oven for 3 days at 65°C.
Quality Control and Quality Assurance
Plastic containers and glasswares used in the experiment were washed with tap water and distilled water then immersed in 2.0 N HNO3 solutions before use. All the standard reagents and chemicals used for atomic absorption spectrophotometer (AAS) analysis were of analytical grade and certified standards. Five replicates samples data of analysis showed a good precision within the results.
Soil and Plant Analyses
Soil Analyses
Soil sample was grind and sieved through 2.0 mm stainless steel mesh model BS 410-1:2000 before the analysis.The experiment was carried out as stated in the procedure outlined by Durumin Iya et al.9 with minor modifications. Exactly 1.0 g of dried soil sample in a crucible was dry-digested (dry ashing) by heating to ash at 500°C for 5.0 h in a Stuart/FX105-27 series muffle furnace. On cooling, 1.0 mL of distilled water and 1.0 mL of concentrated HNO3 acid were added. It was evaporated to dryness on a Favorit HS 0707V2 series hot plate then heated to 400°C for 15 min in a muffle furnace. Immediately 1.0 mL and 2.0 mL of distilled water and concentrated HCl acid, respectively were added then evaporated to dryness. Then 10.0 mL of distilled water was added and filtered with 0.45 micron filiter in 100 mL volumetric flask. Distilled water was added to the mark of 100 mL volumetric flask.
Plant Organs Analyses
Plant organs analysis was performed as stated in the procedure decribed by Durumin Iya et al.9 Plant organs were grind to fine powder and digested before heavy metals analysis. Approximately, 0.5 g of each plant organ (root, stem and leaf) was ashed at 480°C for 4.0 h in a muffle furnace model Stuart/FX 105-27 series. At a low temperature exactly 1.0 mL of distilled water and 1.0 mL of concentrated HNO3 acid were added. It was then evaporated to dryness on a hot plate model Favorit HS 0707V2 series. When cooled, 2.0 mL of 20% (v/v) HNO3 was added then heated to 100°C on hot plate. The digested sample was filtered through 0.45 μm filter paper into 100 mL volumetric flask distilled water was then added to 100 mL mark of the flask. Soil and samples were analyzed on a Perkin Elmer AAS model Optima 8300 series.
Phytoextraction and Phytostabilization Potential of the Plant
Bio-concentration factor (BCF = Metal concentration in tissue of whole plant / Initial concentration of metal in soil) and translocation factor (TF = Shoot metal accumulation/ Root metal accumulation) were the two indices used to calculate the potential of P. fruticose to be a phytoextractor of the heavy metals or phytostabilizer.10,11 Plants are suitable for phytoextraction if they have high BCF values greater than 1, and suitable for phytostabilization if BCF value is high and TF value is low.10
Statistical Analysis
All data reported were averaged values, standard deviation (SD) and standard error of five good replicates selected from eight independent replicates. Statistical analysis of the data was carried out using one-way analysis of variance (ANOVA). Differences in the concentration between harvesting time and between plant organs were considered statistically significant value at p ˂ 0.05. Capital letters were used to indicate the differences in soil and plant organs, while small letters were used to indicate the differences of heavy metals concentration on different harvesting period. The statistical analysis was accomplished by IBM SPSS Statistics version 24.
Results and Discussion
Plant Growth
P. fruticose survived in the soil spiked with several heavy metals. Plant growth was observed to be around 98% and plant was able to adapt the condition of the soil. Moreover, it was observed that the plant survived and grows without any adverse effect or sign of yellowish leaf or undeveloped growth throughout the period. P. fruticose has the potential to uptake 8 heavy metals during first harvest in its roots, these are Co, Cr, Cu, Pb, Fe, Mn, Ni and Zn and were able to translocate some of the heavy metals to the aboveground biomass. Cd and As were not detected by the plant, while the accumulation of Fe was higher compared to other metals.
Heavy Metals Uptake by the Plant Organs
The uptake of heavy metals (Co, Cr, Cu, Ni, Fe, Zn, Mn, and Pb) in the root, stem and leaf tissues of P. fruticose was investigated. A significantly (p < 0.05) high quantities of heavy metals were observed in the root more than the stem and leaf except for Co and Cr which both were accumulated more in the leaf on 120 harvesting day. However, the quantity of Fe were consistently more in soil than in the root, stem and leaf of the plant on all the harvesting days. In this experiment, heavy metals uptake by the roots were in the ranges of (36.88 to 384.19) Cu, (1.99 to 9.24) Co, (12.61 to 68.94) Cr, (24.41 to 226.36) Mn, (21.01 to 56.89) Ni, (14.56 to 193.34) Zn, (3.09 to 21.75) Pb and (23.14 to 499.24) Fe, all in mg/kg. Moreover, the accumulation of heavy metals in the plant organs was significantly (p < 0.05) higher than it’s concentration in the control plant organs.
Cobalt
P. fruticose was able to uptake and accumulates Co from heavy metals spiked soil with a high concentration. As it was shown in figure 1, a significant (p < 0.05) high concentration of Co in the leaf of the plant was observed to be 15.0 ± 6.23 mg/kg on 120 harvesting day, followed by the root on 120 and 240 harvesting days with concentration 7.588 ± 0.43 mg/kg and 9.24 ± 0.85 mg/kg respectively. It was observed that the concentration of Co in soil and plant organs on 300 days were lower than those on 240 days. The concentration of Co accumulated by plant organs was 1 to several folds compared to control soil and plant. Another high accumulation was observed in the stem of the plant on 240 day with concentration 6.77 ± 1.22 mg/kg. In a similar study on Co, Cr and Ni contents in soils and plants from a Serpentinite quarry, the roots of Juncus sp. accumulated a larger amount of Co, both Festuca rubra L. and Juncus sp. L. accumulated Co in the roots more than the shoot,12 while in contrary to this study the accumulation was not consistent. Accordingly, mean concentration of Co was found in the tissues of Luff cylindrica planted on two different soil samples (i) and (ii) with values 0.414 and 0.597 mg/kg, respectively.13 Clethra barbinervis was found to accumulate quiet a number of heavy metals such as Mn, Co, Zn, Ni and Cd but accumulated more of Co and Ni in the leaf.14 It was observed that Ni and Co interacts regularly and acts against each other in the plant.15 Another opposite interaction between Co and Ni was recorded from Alyssum bertolonii and Berkheya coddii and this opposite relationship between Co and Ni may lead to great competition in their translocation, binding ligands and also their function.14 The endurance and accumulation mechanism of Co are not sufficiently understood this could be due to some Co accumulator plants use to uptake and accumulate Cu.14,16 It was reported that a common criteria used to identify a metal hyper-accumulator plant followed the concentration of metal in dried leaf of a plant that grow in a natural way. A hyper-accumulator plant for Co, Cu and Cr should accumulate 300 mg/kg of the metal before being considered as hyper-accumulator.16 Another important issue about Co accumulator is considered to be Co detoxification way will shun the uptake of ions that can cause stress from oxidation.17
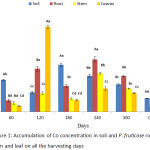 |
Figure 1: Accumulation of Co concentration in soil and P. fruticose root, stem and leaf on all the harvesting days. |
Chromium
Uptake of Cr by P. fruticose grown on a soil contaminated with heavy metals is shown on figure 2. The amount of Cr in P. fruticose was observed to be significantly (p< 0.05) high in the root, stem and leaf on 120 harvesting day. Furthermore, high accumulation was observed from the plant leaf on 120 harvesting day with an amount 98.74 ± 11.97 mg/kg. Other high Cr accumulations were also recorded on the soil, root and stem on 120 harvesting days with a concentration of 50.65 ± 2.87, 68.94 ± 9.97 and 89.74 ± 12.14 mg/kg respectively. It was observed that the accumulation of Cr on 300 harvesting days was lower compared to 240 days accumulation. Accordingly, Cr concentrations in control soil and control plant part were several folds lower than the treated ones. This experiment agrees with a similar study conducted on soil and plants from Serpentinite quarry, it was observed that, the two plants Festuca rubra L. and Juscus species L. were able to accumulate Cr in their roots and shoots. The concentration of Cr accumulated by F. rubra and Juscus species were ranged 13.74 ± 0.63 to 15.37 ± 1.60, 11.21±1.02 to 46.83± 5.91 mg/kg, respectively.12 It was observed that Cr accumulation was significantly higher in the roots than in the shoot. It was also reported that Jatropha curcas accumulated more Cr in the root more than stem and leaf with concentration 27.99±8.10, 15.90±4.52 and 15.17±3.31 mg/kg, respectively.1
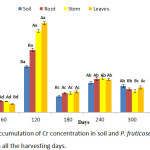 |
Figure 2: Accumulation of Cr concentration in soil and P. fruticose root, stem and leaf on all the harvesting days. |
These results agree with findings of Adki et al.,18 and Rafati et al.,19 both authors found out that Cr concentration is lower at the shoot and vegetables of the plant compared to the root. In another study which is contrary to the present one was found from Portulaca oleracea which have the potential of accumulating higher concentration of Cr >1000 mg/kg,20 and a suggestion was made that any plant that can accumulate Cr more than 1000 mg/kg should be considered as a Cr hyper-accumulator plant.21 The antagonistic relationship and synergistic interactions between the concentration of Cr and other heavy metals in plants tissues are observed.22 It was reported that Cr use to interact with the uptake of other heavy metals such as Fe and Mn in the root and aboveground which eventually reduced organs or tissue concentration.22 In this study, P. fruticose grown on soil spiked with several heavy metals was found to accumulate a significant (p < 0.05) quantity 98.74 mg/kg of Cr dry weight in the leaf. Based on the Cr uptake in this study, P. fruticose cannot be considered as Cr hyper accumulator plant. It was observed that some plants grown on soil treated with wastewater containing Cr, less quantity was transferred to the aboveground plant organs. Moreover, plants that grown on hydrated magnesium silicate soil rich in Cr were found to accumulate few quantities. Generally, plants differ from their potential to absorb and retain Cr in organs and most plants have low capability to uptake and transfer Cr to aboveground tissues.9
Copper
A significant (p < 0.05) high mean value of Cu was observed in the root of P. fruticose plant (384.19 ± 70.69 mg/kg) on 180 harvesting days as presented in figure 3. Other high accumulations were found in the root 42.62 ± 0.55, 36.88 ± 0.96, 69.58 ± 0.85 and 73.18±1.03 mg/kg on 60, 180, 240 and 300 harvesting days respectively. The accumulation of Cu by this plant was observed to be significantly high in the root at all harvesting days. Without considering the harvesting days, all accumulations in the stem and leaf of the plant were far below the concentration in the root.
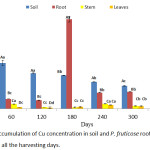 |
Figure 3: Accumulation of Cu concentration in soil and P. fruticose root, stem and leaf on all the harvesting days. |
It was observed in a similar case, where both Brassica napus and Brassica juncea accumulated more Cu in their roots than in shoots under Cu stress for 7 and 14 days in hydroponic solution.23 In contrast, the study carried out on the use of Amaranthus paniculatus and B. juncea for phytoextraction of Pb and Cu from contaminated soil, both plants were able to accumulate Cu concentration ranged from 15.7 to 18.1 and 18.7 to 21.4 mg/kg respectively. But the concentrations were found to be 2 to 4 times more in the shoots than in the roots of the plants.24 Cu is a metal that plants needs for growth, but a concentration of Cu beyond 20.00 mg/kg in the aboveground organs is harmful to the plant.9 In this experiment, Cu accumulation was significantly (p < 0.05) higher in roots 384.19±20.69 mg/kg than stem 21.87±0.67 mg/kg and leaf 16.39±0.98 mg/kg. Higher quantity of Cu in the roots may be due to its ability to move from soil to roots continuously which make it easier for P. fruticose to absorb more and translocate less to aboveground tissue. Deficient transfer of heavy metals to aboveground plant organs could be due to the retaining of heavy metals in protoplasmic fractions of vacuoles, producing coordination complex with some organic acids at the two layers of root or due to separation of heavy metals from anionic sites of cell wall.25
Iron
A significant (p < 0.05) higher accumulation of Fe were observed by P. fruticose in root on 120 harvesting days with concentration of 499.24±29.69 mg/kg as it was shown in figure 4. Other high amount of Fe was found in the stem and leaf on 180 harvesting day with concentration of 382.51 ± 106.71 and 433.08 ± 199.08 mg/kg respectively. Moreover, significant (p < 0.05) Fe accumulations in plant parts were ranged 7.13 to 499.24 mg/kg. Increases of Fe uptake by the root from 60 to 300 harvesting days were also observed with concentration ranged 23.14 to 499.24 mg/kg. All the quantity of Fe accumulated in the P. fruticose organs were significantly (p < 0.05) higher than the control soil and plant parts. This experiment agreed with the study carried out on Camphorosma monospeliacum and Salsola soda for the accumulation of Pb, Fe, Mn, Cu, and Zn in industrial town of Vian. It was reported that the amount of Fe accumulated by the roots of the two plants has a significant difference at the contaminated area with a concentrations ranged 349.6 to 22645.3 mg/kg and 309.6 to 10604.9 mg/kg for root and shoot, respectively, with a maximum concentration in the roots of S. soda and highest amount in shoots of C. monospeliacum.26 In another study conducted on Salvinia cucullata treated with different level of paper mill effluent, the plant accumulated Fe (14,969±1689 mg/kg) in the root and 13,379±1206 mg/kg in the leaf and both were several folds greater than the control.27 In this study, the toxic effect of Cr was not observed, however, a significant high quantity of Fe in the tissues of P. fruticose was observed. There are several factors which increase the performance of the plant in removing Fe from contaminated soil such as the root zone, species of the plant, physical and chemical properties of soil medium, chelating agent addition, environmental condition and the bioavailability of metal.28 Accordingly, a significant reduction (p < 0.05) of Fe quantity in the spiked soil and an increase in Fe accumulations into the plant organs was observed. This indicates that P. fruticose was able to tolerate, uptake and accumulate higher concentrations of Fe from spiked soil medium. Furthermore, plants uptake Fe in the form of ferric (Fe3+) ions and ferrous (Fe2+) ions and make effective use of different Fe absorption mechanisms. The mechanism of some plant in Fe uptake includes the release of proton (H+) and gets reduced by the root of the plant in order to lower the soil pH value. And low pH value at the root zone increases and improves Fe solubility and uptake, respectively.29 Moreover, the metals use to pass through the roots before translocation to the above ground organs of the plant. Plant roots system play an important role in nutrients and water uptake by osmosis from the soil. Higher quantity of Fe uptake through osmosis was transferred to aerial plant organs via xylem.29 And also Fe was translocated via phloem vessels due to some insufficient transpiration flow of xylem vessels in some young plant organs such as apex.29
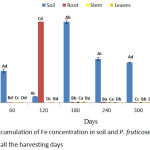 |
Figure 4: Accumulation of Fe concentration in soil and P. fruticose root, stem and leaf on all the harvesting days. |
Manganese
Accumulation of Mn by P. fruticose at the experimental days was presented on figure 5. The plant has the potential to absorb a significant (p < 0.05) concentration of Mn from contaminated soil to the root and translocated it to the shoot. The high accumulation was observed in the root on all the harvesting days with a mean value of 24.42, 101.65, 226.36, 98.68 and 109.97 mg/kg on 60, 120, 180, 240 and 300 experimental days respectively. Furthermore, the amount of Mn accumulated by the plant tissues increases in the trend stem > leaf > roots. The concentrations of Mn accumulated by P. fruticose parts were significantly (p < 0.05) higher than the control plant with several folds. This experiment agreed with the study conducted on Eucalyptus grandis and E. urophylla to identify a Mn hyper-accumulator. Both plants were subjected to the same treatment of hydroponic solution 5.0, 500, 10 000 and 20 000 µM of Mn and were able to accumulate higher concentration in the leaf and stem on the first 3 treatment. On exposure to higher hydroponic solution, a much higher accumulation of Mn was observed in the stem of the plant with concentrations 52,540 mg/kg compared to the leaf and root.30 However, the accumulation of Mn in the stem of the plant at an early stage may be due to the important way of action to prevent the young plant from Mn stress.30 This study is contrary to the experiment conducted by Satpathy and Reddy,31 in which they showed that accumulation of Mn by B. juncea, was higher in stem more than the leaf and root. The ranges of Mn accumulated by the plant tissues were 46.0 to 2041.5, 57.0 to 3781.0 and 50.1 to 3260.8 mg/kg in root stem and leaf, respectively under the same treatment. The amount of Mn accumulated by B. juncea increases in the trend roots > stem > leaf. Moreover, an explanation was made that the quantity of Mn accumulated in the above ground tissues of Mn hyper-accumulator plant is always higher than in the root which shows that the plant has potential to uptake, translocate and keep Mn in the above ground tissues.9
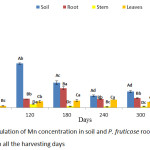 |
Figure 5: Accumulation of Mn concentration in soil and P. fruticose root, stem and leaf on all the harvesting days. |
Nickel
The quantity of Ni accumulated by P. fruticose was presented on figure 6 with a significant (p < 0.05) accumulation in the root on 180 harvesting day with a mean value of 56.89±16.18 mg/kg. Other high accumulations were observed on the stem and leaf on 180 experimental days are with concentrations 43.28 ± 6.71 and 39.55 ± 11.15 mg/kg respectively. All the accumulation in the P. fruticose organs was observed to be significantly (p < 0.05) higher compared to the control plant and soil samples. Moreover, it was observed that the accumulation of Ni in plant parts was higher on 120 and 180 days compared to 240 and 300 days. In a similar study by Roccotiello32 in a Ni phytoremediation potential of the Mediterranean Alyssoides utriculata (L.) Medik, the finding showed that, concentration of Ni accumulated by the leaf of the plant was greater than 1000 µg/g and this classified the plant as a Ni hyper accumulator plant.32 A higher concentration of Ni was observed in the root of P. fruticose at all the harvesting days, followed by the stem on 60 and 120 days. Accumulation of Ni decreases in the trend root > stem > leaf on 60 and 120 experimental days and root > leaf > stem on 180 and 240 harvesting days. In contrast to this experiment, a higher concentration of Ni was found on the stem and leaf of Vetiveria zizanioides more than the root of the plant.33 Uptake of Ni in the root, stem and leaf of P. fruticose was observed to be inconsistent in the trend, instead the accumulation follow the trend root > stem >leaf for 60 and 120 days, while root > stem < leaf for 180 and 240 days.
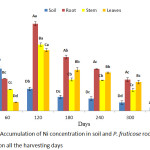 |
Figure 6: Accumulation of Ni concentration in soil and P. fruticose root, stem and leaf on all the harvesting days. |
Furthermore, results indicated a relatively less Ni uptake from root to stem and stem to leaf on 60 and 120 days. P. fruticose was observed to show a high potential to uptake and accumulate Ni in the root on all the experimental days. Salvinia cucullata was found to accumulate Ni in the leaf more when compared to the root in the first 3 treatments applied. It was observed that as the effluent concentration increases the accumulation of Ni in the leaf decreases while at the extreme concentration of the treatment, S. cucullata accumulated more Ni in the root 34.9±0.93 mg/kg.27 Most plants are able to absorb Ni in to the root and translocate it to the above ground tissue by passive and active processes. A high concentration of Ni was observed in the root more than the stem and leaves when plants are grown on soil contaminated with high quantity of Ni. It was observed that excess Ni in the soil may affect the biosynthesis of metalloenzymes by making some important metals inadequate and it also decreases the absorption of Fe by the plant.34
Lead
A mean value of Pb accumulated by P. fruticose was presented on figure 7 and a significant (p < 0.05) high concentrations were observed to increase from 60 to 240 harvesting days and also from root to leaf except for the 60 and 300 experimental days. Furthermore, the accumulation of Pb by P. fruticose parts was several times higher than the concentration in the control plant parts. A significant (p < 0.05) high amount of Pb accumulated by P. fruticose was found in the leaf (96.37 ± 3.89 mg/kg) and least Pb accumulation was also found in the leaf (1.22 ± 0.17 mg/kg) on 240 and 60 harvesting days, respectively. In the experiment conducted on phytoremediation potential for Pb, Zn and Cu using indigenous plants, a range of Pb accumulation was presented as 3.44 to 364.47 mg/kg dry weight in the tissues of the plants.35 This present study agreed with the finding reported by Mkumbo et al.35 The low accumulation of Pb found in this experiment was in accordance with finding reported by Knapp et al.,36 in the Pb uptake by carrots, tomatoes and amaranth with concentrations ranged from 0.3 to 1.9 mg/kg.36 Normally, the plants that accumulated low Pb concentration may be as a result of the toxicity of Pb. Photosynthetic processes, anti-oxidant enzymes and synthesis of chlorophyll can be affected by Pb toxicity.37 Olowu et al.,38 stated that the uptake and high accumulation of Pb at the plant roots may not be translocated to shoots, fruits and seed due to its low solubility and strong barrier.38 Experiment on Pb uptake in P. fruticose shows that leaf has accumulated higher amount of Pb compared to stem and root. Increasing quantity of Pb accumulation in the leaf was found to be 32.85, 61.71 and 96.37 mg/kg on 120, 180 and 240 days, respectively. It was observed that, the amount of Pb accumulated by the tissues of P. fruticose increases from root to stem to leaf. Therefore, the uptake follow the trend root < stem < leaf on all the harvesting days except on the first 60 days. The trend of Pb transfer indicated that Pb was efficiently transferred from the soil to root, root to stem and stem to leaf. The accumulation of Pb into plant organs is an indication of the quantity of Pb contained in the soil or environment. It was observed that small quantity of Pb are soluble and bioavailable for absorption by the plants root from the soil, which could be due to a strong binding between Pb/organic materials and Pb/colloidal materials.39 The potential of plant and cultivars species differs in Pb accumulation, which also depends on Pb availability in the soil and various soil properties.39
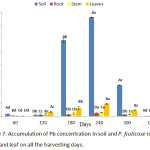 |
Figure 7: Accumulation of Pb concentration in soil and P. fruticose root, stem and leaf on all the harvesting days. |
Zinc
Accumulation of Zn by P. fruticose root, stem and leaf were presented on figure 8. A significant (p < 0.05) concentration of Zn was observed to be higher in the root of the plant at all the harvesting days compared to other plant parts with concentrations 14.56, 58.58, 193.34, 49.25 and 73.27 mg/kg on 60, 120, 180, 240 and 300 days respectively. The accumulation of Zn in P. fruticose part was found higher compared to Zn concentration in control plant part on all harvesting days. A significant (p < 0.05) high amount was found in the root on 120 day with concentration 193.34 ± 36.07 mg/kg. It was observed that, translocation of Zn from root to shoot of the plant decreases in the trend root > stem > leaf at all harvesting days. Other higher amount of Zn in stem and leaf of the plant were found on 180 and 120 harvesting days with a concentration 29.75 ± 4.40 and 27.88 ± 2.21 mg/kg respectively. This study agreed with the similar experiment carried out on Zn uptake using different plant species by Subhashini et al.,40 they reported that Acalypha indica and Ruellia tuberosa accumulated Zn in the tissues following the trend root > stem > leaf.
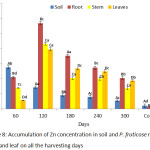 |
Figure 8: Accumulation of Zn concentration in soil and P. fruticose root, stem and leaf on all the harvesting days. |
A. indica was able to accumulate Zn 131.36, 53.93 and 49.92 mg/kg and R. tuberosa accumulated 49.99, 44. 86 and 32.77 mg/kg both for the root, stem and leaves respectively on 60 experimental day.40 In contrary to this experiment, Limnobium laevigatum was found to accumulate a significant concentration of Zn in the leaf with the Zn increase. They also reported that there were no significant changes in the accumulation of Zn from the root and leaf over a period of time.41 High Zn concentration (193.34 mg/kg) was found in the root on 180 days followed by (58.58mg/kg) on 120 days and (49.25mg/kg) on 240 days. Zn is an essential metal to plant in which a normal plant can have a Zn average value of 66.0 mg/kg in the stem or leaf.9 In this study the concentration in the stem and leaf were below the normal plant Zn concentration. However, the uptake of Zn from root to stem and stem to leaf were found to be decreasing on all the harvesting days. Consequently, Zn concentration was found significantly higher in the root than the stem and leaf. Moreover, transfer of Zn from root to stem and leaf was lower compared to transfer from soil to the root of the plant. It was reported that, when Zn is effective it can move and transfer from plant root to shoot and becomes accountable for lowering the amount of important plants nutrients and hinder some biochemical reaction.42 It was also reported that in order to translocate Zn from root to the shoot, Zn has to move through many different tissues before the xylem of the plant was reached.43 But in this experiment Zn was not transferred to aboveground tissue so easily. Accordingly, Zn is capable of moving and it is accessible biologically, consequently, its high amount in the soil and plant can enter in to the food chain.9
Bio-concentration Factor (BCF), Translocation Factor (TF) and Extraction Coefficient (EC)
BCF, TF and EC values obtained are presented in Figures 9, 10 and 11 respectively. The threshold value for BCF > 1 was obtained by many heavy metals on different harvesting days. The high BCF value was achieved by Ni with 9.77, 9.09 and 9.05 values for 120, 180 and 240 harvesting days respectively. Other high values were achieved by Cr (5.08), Co (7.55) and Pb (7.09) on 120 harvesting day. Cr and Ni achieved a BCF value of >1 in the entire harvesting days of 60,120,180 and 240, while Fe was not able to achieve the BCF threshold value. Minimum value was observed in Fe on 60 harvesting day with BCF value of 0.002. High and low BCF values of 5.57 and 0.02 were observed in Sphaeranthus africanus (L.) and Celosia triggna L. respectively35 and this is similar to the present experiment. The TF threshold of >1 was observed in Co, Cr, Ni, Mn, Zn, Fe and Pb heavy metals at different experimental days except Cu which did not achieve TF value >1 on all the experimental days. Cr was observed to achieved TF threshold of >1 on all the harvesting days. It was observed that Pb has the highest TF value on 120, 180 and 240 harvesting days compared to other metals. Cu failed to be efficiently translocated to the shoot when compared to other heavy metals, and Fe was poorly translocated. The highest TF values were observed at different experimental days, Cr (TF = 2.73), Pb (TF = 7.37), Pb (TF = 5.7) and Pb (TF = 5.29). Therefore, these results indicated that P. fruticose is an excluder species to Cu and Fe while it is an accumulator to Co, Cr, Ni, Mn, Pb and Zn.
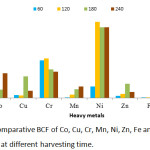 |
Figure 9: Comparative BCF of Co, Cu, Cr, Mn, Ni, Zn, Fe and Pb in P. fruticose at different harvesting time. |
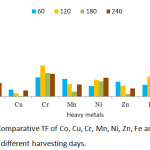 |
Figure 10: Comparative TF of Co, Cu, Cr, Mn, Ni, Zn, Fe and Pb in P. fruticose at different harvesting days. |
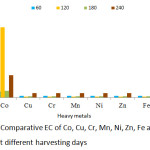 |
Figure 11: Comparative EC of Co, Cu, Cr, Mn, Ni, Zn, Fe and Pb in P. fruticose at different harvesting days. |
The EC threshold value of >1 was obtained by Ni and Cr heavy metals on all the harvesting days. Maximum EC was achieved by Pb on 120 days with value 5.96 followed by Ni on 120, 180 and 240 harvesting days with values 5.79, 5.21 and 5.63 respectively. Co also obtained a maximum value of 5.34 on 120 experimental days, while the remaining metal shows EC < 1 throughout the harvesting days.
Conclusion
Soils that are polluted with heavy metals or metalloid are not easy to be remediated. The present practice of soil clean-up is very expensive; therefore a low cost technology is needed for rectifying contaminated soil. A recent technology called phytoremediation is cost effective and affordable for reducing or removing heavy metals and metalloids from contaminated soil. In this study, growth, survival ability, accumulation of heavy metals in the roots, stem and leaf, the relationship between the metal concentration in the soil, root and shoots were the factors of concern in conducting the experiment. According to the experimental results obtained, P. fruticose was a good accumulator for most of the heavy metals because the concentration ratio of the heavy metals in the plant to that in the soil (BCF) was > 1. P. fruticose plant has the potential to be used in the phytoextraction of some heavy metals with BCF and TF > 1. Furthermore, it has the ability to be used in phytostabilization for the heavy metals with BCF and TF values < 1. The results obtained for this experiment are considered to be used as a practical demonstration for the use of P. fruticose in the phytoremediation of several heavy metal contaminated soils and it’s the novelty of the experiment.
All authors contributed in carrying out the experiments and writing of the manuscript. All authors read and approved the final manuscript.
Conflict of Interest
There is no conflict of interest.
Acknowledgments
The authors would like to show gratitude to the Faculty of Resource Science and Technology, Universiti Malaysia Sarawak, and the study leave granted to Naseer Inuwa Durumin Iya by Federal University Dutse, Nigeria for his Ph.D is also acknowledged.
References
-
Chang, F.C.; Ko, C.H.; Tsai, M.J.; Wang, Y.N.; Chung, C,Y. Ecotox. 2014, 23(10), 1969–1978.
CrossRef - Ullah, A.; Henga, S.; Munis, M. S. H.; Fahad, S.; Yanga, X. Environ and Exp Bot. 2015, 117, 28–40.
CrossRef - Pandey, V. C. Ecotox. Environ. Safe. 2012, 82, 8–12
CrossRef - Iqbal, M. P. Pak J Pharm Sci. 2012, 25, 289–294.
CrossRef - Wuana, R. A.; Okieimen, F. E. Ecol. 2011, 1-20.
CrossRef - Beskoski, V. P.; Gojgic-Cvijovic, G.; Milic, J.; Ilic, M.; Miletic, S.; Solevic, T.;Vrvic, M. M. Chemos. 2011, 83, 34-40.
CrossRef - Sawidis, T.; Krystallidis, P.; Veros, D.; Chettri, M. Bio Trace Element Resour. 2012, 148(3), 396-408.
CrossRef - Singh, R.; Singh, D. P.; Kumar, N.; Bhargava, S. K.; Barman, S. C. J Environ Bio. 2010, 31, 421- 430.
- Durumin Iya, N. I.; Assim, Z.B.; Ipor, I.I.; Omolayo, A.O.; Umaru, I.J.; Jume, B.H. Indones. J. Chem. 2018, 18(3), 503–513.
CrossRef - Karam, D.; Rajoo, K.; Ismail, A.; Muharam, F.M. Am. J. Agric. Environ. Sci. 2016, 16(8), 1504–1514.
- Kaewtubtim, P.; Meeinkuirt, W.; Seepom, S.; Pichtel, J. Appl. Ecol. Environ. Res. 2016, 14(1), 367– 382.
CrossRef - Lago-Vila, M.; Arenas-Lago, D.; Rodríguez-Seijo, A.; Andrade Couce, M. L.; Vega, F. A. Solid Earth, 2015, 6, 323–335.
CrossRef - Khan, Z. I.; Ahmad, K.; Ashraf, M.; Parveen, R.; Mustafa, I.; Khan, A.; Bibi, Z.; Akram, N. A. Pak. J. Bot. 2015, 47(1), 217-224.
- Yamaguchi, T.; Tomioka, R.; Takenaka. Int. J. Mol. Sci. 2015, 16, 21378-21391.
CrossRef - Kabata-Pendias, A. Trace Elements in Soils and Plants, 2011, 227–251.
CrossRef - Ali, H.; Khan, E.; Sajad, M. A. Chemos, 2013, 91, 869–881.
CrossRef - Lange, B.,; van der Ent, A.; Baker, A. J. M.; Echevarria, G.; Mahy, G.; Malaisse, F.; Meerts, P.; Pourret, O.; Verbruggen, N.; Faucon, M. P. New Phytol, 2016, 213, 537 – 551.
CrossRef - Adki, V. S.; Jadhav, J. P.; Bapat, V. A. Environ. Sci. Pollut. Res. 2013, 20, 1173-1180.
CrossRef - Rafati, M.; Khorasani, N.; Moattar, F.; Shirvany, A.; Moraghebi, F.; Hosseinzadeh, S. Intl. J. Environ. Res. 2011, 5, 961–970.
- Tayim, A.; Alyazouri, A.; Jewsbury, R. A.; Tayim, H.; Humphreys, P. N.; Al-Sayah, M. H. Toxicol. Environ. Chem. 2014, 95(8), 1029–486.
CrossRef - Ali, H.; Naseer, M.; Sajad, M. A. Int. J. Environ. Sci. 2012, 2, 1459–1469.
- Emamverdian, A.; Ding, Y.; Mokhberdoran, F.; Xie, Y. The Sci. World J. 2015, 1-18.
CrossRef - Feigl, G.; Kumar, D.; Lehotai, N.; Tugyi, N.; Molnar, A.; Ordog, A.; Szepesi, A., Gemes, K.; Laskay, G.; Erdei, L.; Kolbert, Z. Ecotoxicol. Environ. Safe, 2013, 94, 179–189.
CrossRef - Rahman, M. M.; Tan, P. J.; Faruq, G.; Sofian, A. M.; Rosli, H.; Boyce, A. N. Int. J. Agri. c Bio. 2013, 15, 903–908.
CrossRef - Leura-Vicencio, A.; Alonso-Castro, A. J.; Carranza-Alvarez, C.; Loredo-Portales, R.; Torre, M. C. A.; Cruz, R.F. G. Bull. Environ. Cont. Toxicol, 2013, 90, 650–653.
CrossRef - Lorestani, B.; Cheraghi, M.; Yousefi, N. Arch. Bio. Sci. 2011, 63(3), 739 –745.
CrossRef - Das, S.; Mazumdar, K. Chemos, 2016, 163, 62–72.
CrossRef - Tangahu, B.V.; Abdullah, S.R.; Basri, H.; Idris, M.; Anuar, N.; Mukhlisin, M. Int. J. Chem. Eng. 2011, 1-31.
CrossRef - Bhat, I. U.; Mauris, E. N.; Khanam, Z. Int. J. Phytorem. 2016, 18(9), 918-23.
CrossRef - Xie, W.; Huang, Q.; Li, G.; Rensing, C.; Zhu, Y, Int. J. Phytorem. 2013, 15, 385–397.
CrossRef - Satpathy, D.; Reddy, M. V., Appl. Ecol. Environ. Res. 2013, 11(4), 661 –679.
CrossRef - Roccotiello, E.; Serrano, H. C.; Giorgio, M.; Branquinho, M. C. Chemos, 2015, 119, 1372–1378.
CrossRef - Gunwal, I.; ingh, L.; Mago, P. Int. J. Sci. Res. Pub. 2014, 4(10), 1–7.
- Nie, J.; Pan, Y.; Shi, J.; Guo, Y.; Yan, Z.; Duan, X.; Xu, M. Int. J. Environ. Res. Pub. Health, 2015, 12, 15075–15087.
CrossRef - Mkumbo, S.; Mwegoha, W.; Renman, G. Int. J. Environ. Sci. 2012, 2(4), 2425–2434.
- Knapp, L.; Sangster, J.; Bartelt-hunt, S. L. Int. J. Serv. Earn. Eng. 2013, 8(2), 1-7.
CrossRef - Nematian, M.A.; Kazemeini, F. Intl. J. Agric. Crop Sci. 2013, 5(4), 426–432.
- Olowu, A. R.; Adewuyi, O. G.; Onipede, J. O.; Oladipo, A.; Lawal, A.O.; Owolabi, M.; Sunday, M. O. Am J Chem. 2015, 5(1), 40–48.
- Amin, H.; Arain, B.A.; Jahangir, T.M.; Abbasi, M.S.; Amin, F. Geology, Ecology, and Landscapes, 2018, 2(1), 51-60.
CrossRef - Subhashini, V.; Swamy, A.V.V.S.; Krishna, R. H. World. J. Environ. Eng. 2013, 2, 27-33.
- Aran, D.S.; Harguinteguy, C.A.; Fernandez-Cirelli, A.; Pignata, M.L. Environ. Sci. Pollut. Res. Int. 2017, 24(22), 18295-18308.
CrossRef - Sheel, D. R.; Anand, M.; Nisha, K. Intl. J. Sci. Res. 2013, 4(7), 1238–1241.
- Gupta, N.; Ram, H.; Kumar, B. Rev. Environ. Sci. Biotech. 2016, 15, 89–109.
CrossRef

This work is licensed under a Creative Commons Attribution 4.0 International License.









