Phytoremediation of Chromium from Electroplating Effluent Treatment Plant (Etp) Sludge Using Helianthusannuus L.
Department of Biotechnology, Karpagam Academy of Higher Education, Eachanari post, Coimbatore 641021, Tamil Nadu, India.
Corresponding Author E-mail id:rajivsmart15@gmail.com
DOI : http://dx.doi.org/10.13005/ojc/340638
Article Received on : 19-07-2018
Article Accepted on : 26-11-2018
Article Published : 29 Nov 2018
Phytoremediation is one of the best methods in the treatment of sludge from industries because the pollutants present in the sludge are the food source for plants. So, the present study dealt with wedging the electro-plated sludge with red soil for 30 days and using it for the growth of Helianthus annuus L. The sludge and soil were blended in different concentrations C1, C2, C3, C4, C5 and C6 (for the pre-treatment). Physicochemical parameters (pH, ammoniacal nitrogen, potassium and phosphate) and heavy metals (Ar, Cd, Pb, Hg, Ni and Cr) of raw sludge, soil and ETP-treated-sludge with soil were analysed. After inspecting the physicochemical parameters of raw sludge and treated sludge, the soil-treated-sludge was used for the growth of Helianthus annuus L. Phytoremediation by Helianthus annuus L. has made considerable changes in the physicochemical properties of the soil, specially denoting the reduction of chromium. Thus, the work concludes that the pre-treated sludge provides a pathway for the uptake of heavy metals through the process of phytoremediation.
KEYWORDS:Chromium; Helianthus annuus L.; Phytoremediation; Sludge
Download this article as:| Copy the following to cite this article: Suresh M. J, Rajiv P. Phytoremediation of Chromium from Electroplating Effluent Treatment Plant (Etp) Sludge Using Helianthusannuus L. Orient J Chem 2018;34(6). |
| Copy the following to cite this URL: Suresh M. J, Rajiv P. Phytoremediation of Chromium from Electroplating Effluent Treatment Plant (Etp) Sludge Using Helianthusannuus L. Orient J Chem 2018;34(6). Available from: http://www.orientjchem.org/?p=53240 |
Introduction
The development of technology and industries leads to the production of new materials and products, which cause pollutions that are very harmful to the environment. The wastes produced by the industries cause numerous problems to soil and water resources. These wastes contain heavy metals like cadmium, zinc, lead, chromium, nickel, copper, vanadium, platinum, silver, and titanium.1 Most of the wastes from industries are let into the soil and water courses directly. The wastes are degradable and non-degradable. Some microorganism can degrade the wastes,which takes a long time. Some toxic wastes are non-degradable. Heavy metals are important for living things at low concentration for their growth and metabolism. At high levels heavy metals are harmful, toxic, carcinogen and allergen. They can render inactive sensitive enzymes.2
Chromium (Cr) is present in most of the industrial wastes (textiles, tanneries, steel and cement manufacturing industries) that are directly let into the environment affecting the health of living organism.3 Concentration Cr in soil is 0.1 to 250 ppm or even 400ppm depending on the geographical area.4 The stable forms of Cr in environment are tri and hexavalent. These (Cr) are obtained from industrial wastes to environment(Halides, oxides and sulphides).5
The disposal of untreated wastes from industries increases heavy metal levels in the soil and health issues.6 Nowadays it is one of the most uncontrollable problems for farmers. So, a proper technique is needed to control metals in soils.7 Solidification, stabilization, landfill, excavation, vitrification, incineration, washing, flushing and electro kinetic systems are the methods used to control the heavy metals in the environment which have their own specificity and limits.8
Phytoremediation is the best technique to solve this problem which is eco-friendly and cost-effective.9 In this method heavy metals are eliminated or controlled by plants.10 The methods involved in Phytoremediation are stimulation of microbes, degradation, accumulation or extraction, volatilization and stabilization.11 When compared to others it is a low cost green technology.12 The application of this method cleans the soil and water and the residues can be recycled without causing much damage to the environment.13,14 In this present investigation electroplating effluent treatment plant (ETP) sludge was treated using Helianthus annuus.
Materials and Methods
Collection of Electroplated Sludge and Analysis of Physicochemical Parameters
Electroplated sludge was collected from the Lakshmi Machine Works (Latitude 10.603 and Longitude 77.378) located adjacent to Kaniyur village, Coimbatore. The collected sludge was stored at room temperature on PVC bags for a day. By the next day, physicochemical parameters of the sludge such as pH, ammoniacal nitrogen, potassium, phosphate and heavy metals (Ar, Cd, Pb,Cr, Hg, and Ni) were assessed using standard methods.15
Collection of Seedsand Soil
The seeds of Helianthus annuus were collected from the Department of Oil Seeds, Agricultural University, Coimbatore. Red soil was collected from 0–5 cm depth on the field of Kenathukadavu (10.82ºN, 77.02 ºE), total area 9 km2, Coimbatore.
Pre-treatment
The collected electroplating sludge was pre-treated with red soil in the following ratio. The control (C1) is pure red soil, soil and sludge in the ratio of 8:2 (C2), soil and sludge in the ratio of 6:4 (C3), soil and sludge in the ratio of 4:6 (C4), soil and sludge in the ratio of 2:8 (C5) and the collected sludge (C6). The physicochemical parameters of soil, pre-treated sludge (C2, C3, C4 and C5) and the sludge (C6) were analysed after the incubation period of 30 days. This pre-treated sludge was further used for the growth of Helianthus annuus L. Ten seeds of Helianthus annuusweresown on a 12L clay pot which was filled with pre-treated sludge. The percentage of seed germination was measured after 7 and 14 DAS. The plant growth (root length, shoot length, dry weight and fresh weight of plant) was determined after 30, 60 and 90 DAS. Table 1 shows the physicochemical properties of soil and ETP sludge.
Table 1: Treatment of ETP mixed with red soil.
| Treatments |
Particulars |
| Treatment-1 (C1) | Red soil 10 Kg (control) |
| Treatment-2 (C2) | 8 Kg of Red soil + 2 Kg of ETP |
| Treatment-3 (C3) | 6 Kg of Red soil + 4 Kg of ETP |
| Treatment-4 (C4) | 4 Kg of Red soil + 6 Kg of ETP |
| Treatment-5 (C5) | 2 Kg of Red soil + 8 Kg of ETP |
| Treatment-6 (C6) | 10 Kg of ETP |
Estimation of Chlorophyll
Chlorophyll (aandb) was estimated according to Arnon, 1949 (16). 5gram of fresh plantleaf sample was taken and homogenized in tissue homogenizer with 10 ml of extractant (methanol). 10 mL of the leafextract was centrifuged at 10,000 rpm for 10 min. The collected pellet was mixed with methanol. The mixture was centrifuged at 5000 rpm for 10 min; the absorbance value of supernatant was measured using UV-spectrometer (UV–2450, Shimadzu) at 663 nm (Chlorophyll a) and 645 nm (Chlorophyll b).
Analysis of Physicochemical Properties
10 gramsof treated and untreated sludge was taken and centrifuged at 10,000 rpm for 20 min; the pellet was discarded and the pH of supernatant was analysed by pH meter (ELICO Model-107). The assessment of pH, ammoniacal nitrogen, potassium and phosphate were followed by APHA16 method. Heavy metals (Ar, Cd, Pb, Hg, Ni and Cr) were assessed using Atomic Absorption Spectrophotometer (1983-400 HGA 900/AS 800 Perkin Elmer) and multi-Element Standard (MERCK-112837).17 Table 2 and 3 show the physicochemical parameters of raw sludge, red soil and pre-treated ETP sludge.
Table 2: Physicochemical properties of ETPbefore treatment.
| S.No |
Parameters |
Red soil |
ETP |
|
1 |
pH | 7.3±0.01 | 7.7±0.02 |
|
2 |
Ammoniacal Nitrogen (mg/kg) | 10.45±1.26 | 95±0.89 |
|
3 |
Phosphate (mg/kg) | 12.68±0.56 | 86±0.92 |
|
4 |
Potassium (mg/kg) | 0.15±0.06 | 26±0.18 |
|
5 |
Arsenic (mg/kg) | 0.05±0.01 | 32.12±1.64 |
|
6 |
Cadmium (mg/kg) | 5.3±0.26 | 47.2±2.23 |
|
7 |
Lead (mg/kg) | 0.03±0.0 | 89.75±3.21 |
|
8 |
Mercury (mg/kg) | 0.10±0.18 | 1.10±0.26 |
|
9 |
Nickel (g/kg) | ND | 49.56±5.10 |
|
10 |
Chromium (g/kg) | ND | 313.1±3.36 |
Table 3: Physicochemical properties of ETP pre-treated sludge after 30 days.
| S.No |
Parameters |
Treatments |
|||||
|
C1 |
C2 |
C3 |
C4 |
C5 |
C6 |
||
|
1 |
pH | 7.3±0.10 | 7.85±0.12 | 7.66±0.12 | 7.63±0.06 | 7.60±0.05 | 7.7±0.02 |
|
2 |
Ammoniacal Nitrogen (mg/kg) | 10.45±1.26 | 62±1.06 | 74±1.58 | 86±1.66 | 90±1.78 | 95±0.89 |
|
3 |
Phosphate (mg/kg) | 12.68±0.56 | 53±1.31 | 61±1.56 | 68±1.88 | 75±1.91 | 86±0.92 |
|
4 |
Potassium (mg/kg) | 0.15±0.06 | 12±0.10 | 16±0.25 | 19±0.36 | 20±0.51 | 26±0.18 |
|
5 |
Arsenic (mg/kg) | 0.05±0.001 | 15±0.09 | 20±0.59 | 26±0.65 | 28±0.68 | 32.12±1.64 |
|
6 |
Cadmium (mg/kg) | 5.3±0.26 | 32±0.40 | 35±0.59 | 38±1.10 | 41±1.35 | 47.2±2.23 |
|
7 |
Lead (mg/kg) | 0.03±0.001 | 68±0.73 | 72±1.21 | 76±1.38 | 80±1.51 | 89.75±3.21 |
|
8 |
Mercury (mg/kg) | 0.10±0.001 | 0.12±0.001 | 0.22±0.001 | 0.10±0.001 | 0.18±0.001 | 1.10±0.01 |
|
9 |
Nickel(g/kg) | ND | 43.22±6.21 | 44.41±4.34 | 45.58±5.16 | 46.99±4.28 | 49.56±5.10 |
|
10 |
Chromium (g/kg) | ND | 286.1±3.10 | 306.2±4.240 | 308.6±2.450 | 310.8±2.10 | 313.1±3.36 |
Statistical Analysis
Experiments were carried out with triplicates. Results are presented with means ± standard errors for all the three experiments.
Analysis of Seed Germination and Plant Growth
Ten numbers of seeds were soaked in a separate pot under 1 cm depth. These seeds were then spilled with water. Table 4 shows the percentage of the measurement of seed germination all the pre-treated sludge (C1, C2, C3, C4, C5 and C6). No fertilizers were used. Shoot and root length, dry and fresh weight of plant were measured after 30, 60, and 90 DAS. After the flowering stage, numbers of petals were counted after 45 DAS. On the 90th day, weight of a seed per plant and the total no of 25 seeds per plant were measured.18
Table 4: Germination of seeds on the pre-treated ETP +Soil.
| Treatments |
Seed Germination percentage (%) |
|
| 7th DAS | 14th DAS | |
| Treatment-1 (C1) | 98 | 98 |
| Treatment-2 (C2) | 80 | 85 |
| Treatment-3 (C3) | 50 | 55 |
| Treatment-4 (C4) | 25 | 28 |
| Treatment-5 (C5) | 12 | 13 |
| Treatment-6 (C6) | 0 | 0 |
Results and Discussion
Table 5 shows the physicochemical parameters of post-harvested soil after the phytoremediation with Helianthus annuus. In the pre-treated sludge, there was less variation in pH from 7.3 to 7.85 which resulted inhigh metal binding with soil, thereby achieving further extraction of metal through phytoremediation.According to Chaney et al.,19 due to pre-liming of soil phytoextraction of metals had been reduced. In the treatment of C2 and C3, there was a reduction of potassium (12mg/kg, 16mg/kg) and phosphate (53 mg/kg, 61 mg/kg), but in C4 and C5, the micro-organisms in the soil were unable to uptake these minerals, due to the high concentration of sludge.20 Helianthus annuuswas able to uptake Pb and Ni from ETP mixed soil (72 mg/kg and 44,410 mg/kg), which was quite low comparedto the plant Catharanthusroseus which up tookPb and Ni by 67.34 mg/kg and 45.75 mg/kg.21 Helianthus annuuswas able to survive and uptake chromium (183704 mg/kg) from pre-treated sludge. Cynodondactylon (L.) Pers., Vetiverianemoralis (A.) Camus, Echinochloacolonum (L.) Link., Phyllanthus reticulates Poir., Plucheaindica Less., and Amaranthusviridis were removes low amount of chromium from the soil.22 From the pre-treated sludge,Helianthus annuuswas able to remove 12% of Cd, similarly to the plant of Thlaspicaerulescens [23]. Production of chlorophyll was above 40% in the presence of chromium which was similar to the plant Sorghum.24
Table 5: Physicochemical properties of post harvested soil (1st stage) (Chromium removal).
| S.No |
Parameters |
Treatments |
||||
|
C1 |
C2 |
C3 |
C4 |
C5 |
||
|
1 |
pH | 7.03±0.01 | 7.06±0.09 | 7.08±0.12 | 7.07±0.15 | 7.66±0.17 |
|
2 |
Ammoniacal Nitrogen (mg/kg) | 7.75±0.21 | 58±1.22 | 66±2.26 | 71±2.10 | 78±2.09 |
|
3 |
Phosphate (mg/kg) | 8.21±0.5 | 47±1.55 | 56±1.58 | 63±2.21 | 72±2.35 |
|
4 |
Potassium (mg/kg) | 0.03±0.001 | 9±0.06 | 12±0.36 | 15±0.85 | 18±0.77 |
|
5 |
Arsenic (mg/kg) | 0.02±0.001 | 10±0.81 | 16±0.88 | 20±1.09 | 24±1.10 |
|
6 |
Cadmium (mg/kg) | 3.13±0.12 | 27±1.26 | 30±1.41 | 33±1.36 | 39±1.58 |
|
7 |
Lead (mg/kg) | 0.02±0.001 | 60±1.64 | 68±1.76 | 70±2.12 | 77±2.35 |
|
8 |
Mercury (mg/kg) | 0.05±0.001 | 0.10±0.001 | 0.15±0.001 | 0.09±0.001 | 0.12±0.01 |
|
9 |
Nickel (g/kg) | ND | 40.57±1.54 | 42.37±2.68 | 44.45±3.15 | 45.87±1.22 |
|
10 |
Chromium (g/kg) | ND | 110.8±1.36 | 122.5±2.76 | 202.5±3.46 | 301.8±2.36 |
Figure 1 and 2 show fresh and dry weight of plants that were analysed after 30th, 60th and 90th day of seedling. When compared to all the six treatments control (C1) showed more significant result than C2, C3, C4 and C5. The maximum weight was observed in C2 and C3 treatment. Minimum weight was observed in C5. There was no growth in the treatment C6.Maximum lethal of plants occurred in higher concentration of chromium than in the lower concentration. This might be the result ofdeficiency of nutrients and water uptake.25 Fresh and dry weight of Helianthus annuus the soil mixed with ETP sludge was higher compared with rice plants as reported by Sharma25 and grown rice plants on Cr contaminated soil.
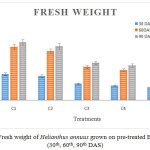 |
Figure 1: Fresh weight of Helianthus annuus grown on pre-treated ETP sludge (30th, 60th, 90th DAS). |
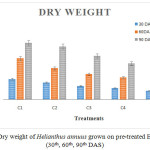 |
Figure 2: Dry weight of Helianthus annuus grown on pre-treated ETP sludge (30th, 60th, 90th DAS). |
Figure 3 and 4 show the root and shoot length of plants that were decreased according to the concentration of Chromium. In high concentration of chromium, the length of roots and shoots were less. A significant result was observed in control C1 than C2 and C3. Minimum root and shoot length was observed in C5 treatment. The results of root and shoot length of Helianthus annuuswere identical with the tomato and brinjal plants, showing less growth with Cr concentration of 200 mg/L.26
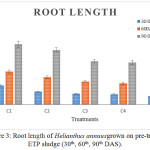 |
Figure 3: Root length of Helianthus annuusgrown on pre-treated ETP sludge (30th, 60th, 90th DAS). |
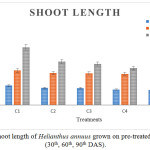 |
Figure 4: Shoot length of Helianthus annuus grown on pre-treated ETP sludge (30th, 60th, 90th DAS). |
Figure 5 and 6 show the weight of a seed per plant and weight of 25 seeds per plant that were reduced gradually based on the concentration of heavy metals. Treatment C1 showed maximum weight of seeds when compared to others. C5 showed minimum weight of seeds which was high in heavy metal concentration (Ar, Cd, Pb, Hg, Ni and Cr). The Concentration of Chromium in sludge was higher than other heavy metals. Nitrogen was a part of biomolecules (proteins and nucleic acids), hence deficiency of nitrogen prohibited the growth and yield of plants.27
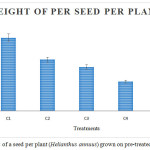 |
Figure 5: Weight of a seed per plant (Helianthus annuus) grown on pre-treated ETP sludge. |
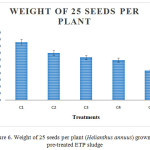 |
Figure 6: Weight of 25 seeds per plant (Helianthus annuus) grown on pre-treated ETP sludge. |
Conclusion
The present investigation concluded that the Helianthus annuus could be used for the removal of chromium from the ETP sludge. The ETP sludge might improve the growth and yield of Helianthus annuus. This technology could be eco-friendly, pollution free and cheap.
Acknowledgements
We are thankful to Karpagam Academy of Higher Education, Coimbatore for providing the support to carryout the research.
References
- Mathur, A.K.; Iijima, K.K.; Tandon, S.K.; Dikshith, T.S.; Bull. Environ. Contam. Toxicol. 1977, 17, 241-248.
CrossRef - Koropatnick, J.; Leibbrandt, M.E.I.; Biochem. AspectsSpringer, 1995, 115, 93-120.
- Ajmal, M.; Rao, W.A.; Peyton, J.N.; Bioremed. J., 1996,6, 205-215.
- Langard, S.; Internat. Arch. of Occup. Environ. Heal., 1980, 46, 1-9.
CrossRef - U.S. Environmental Protection Agency Washington, DC; 1998, (CAS No. 16065-83-1).
- Carmina, G.; Roc, R.; Antonio, H.; David, W.; Bernal, M.; Ramon, S.; Juan, N.; Biochem. Biophys. Res. Commun. 2003, 303, 440-445.
CrossRef - David, S.; Michael, B.; Nanda, K.; Viatcheslav, D.; Burt, E.; Ilan, C.; Ilya, R.; Nat. Biotechnol. 1995, 13, 468 – 474.
CrossRef - MADEP (Massacgysetts Department of Environmental Protection Publication) 1993: Massachusetts Contingency Plan (MCP), Boston.
- Singh, O.; Jain R.; Appl. Microbiol. Biotechnol. 2003,63, 128–135.
- Ilya, R.; Robert, S.; David, S.; Curr. Opin. Biotechnol. 1997, 8, 221–226.
CrossRef - Elizabeth, P.; Pub. Med. Web. Sci. 2005,56, 5-39.
- Prasad, M.N.V.; Freitas H.; Electron. J. Biotechnol. 2003, 6, 285-321.
CrossRef - Schnoor, J.L.; Technol. Eval. Report. 2002, 1, 252-630.
- Alireza, H.; Farhang, M.; Iran. J. Plant Phy. 2010,2, 95-99.
- Clesceri, L.S.; Greenberg AE.; Trussel, R.R.; 1989, American Public Health Association, Washington, DC.
- Arnon DI.; Plant physiol. 1949,24, 1-15.
CrossRef - Fraile A.; Penche S.; González F.; Chem. Ecol. 2005,21, 61-75.
CrossRef - Francis E.;I.J.S.E.R. 2017, 1, 1751–1757.
- Chaney, R.L.; Li, Y.M.; Angle, J.S.; Baker, A.J.M.; Reeves, R.D.; Brown, S.L.; Homer, F.A.; Malik, M.; and Chin, M.;Phytoremediation of contaminated soils and water. CRC Boca Raton, FL: CRC press; 2000,131–160.
- Rauser W. E.; Cell Biochem. Biophys. 1999, 31, 19-48.
CrossRef - Subhashini, V.;Swamy. A.V.V.S.; U.J.E.R.T. 2013, 3, 465-472.
- Pantawat S.; Wasant P.; Sutha K.; Eakalak K.; Water, Air, Soil Pollut. 2006,6,191–206.
CrossRef - Gerard, E.; Echevarria, G.; Sterckeman, T.; Morel, J.L.P.; J. Environ. Qual. 2000,29, 1117-1123.
CrossRef - Revathi, K.; Haribabu, T.E.; Sudha. P.N.; I.J.E.S.T. 2011,2, 417–428.
- Sharma, D.C.; Mehrotra, S.C.; Indian J. Environ. Health.1993, 35, 330-342.
- Purohit, S.; Varghese, T.M.; Kumari, M.; Indian J. Plant Physiol. 2003, 8, 17–22.|
- Chatterjee, J.; Chatterjee, C.; Environ. Pollut. 2000, 109, 69–74.
CrossRef

This work is licensed under a Creative Commons Attribution 4.0 International License.










