Macro Coumarins as Novel Antioxidants
Dunya L. AL-Duhaidahawi 1 , Yasameen K. Al-Majedy2, Hiba H. Ibraheem2, Abdul Amir H. Kadhum3 and Ahmed A. Al-Amiery2
, Yasameen K. Al-Majedy2, Hiba H. Ibraheem2, Abdul Amir H. Kadhum3 and Ahmed A. Al-Amiery2
1Department of Chemistry, Pharmaceutical College of Pharmacy, University of Kufa, AL-Najaf 31001, Iraq.
2Department of Chemistry, Branch of Applied Science, University of Technology (UOT), Baghdad 10001, (Y.K.A); Iraq.
3Department of Chemical and Process Engineering, University Kebangsaan Malaysia (UKM), Bangi, Selangor 43000, Malaysia.
Corresponding Author E-mail: dunyal.mohammed@uokufa.edu.iq
DOI : http://dx.doi.org/10.13005/ojc/340544
Article Received on : 29-03-2018
Article Accepted on : 25-08-2018
Article Published : 10 Oct 2018
The present work pointed at the design and synthesis of novel coumarin compounds as antioxidants. The alteration of 4-hydroxycoumarin by several reaction levels was performed to generate destination compounds. Spectroscopic methods like Infrared Spectra FT-IR, 1H-NMR and 13CNMR, elemental analysis techniques, melting point, and thin layer chromatography defined the structure of the prepared compounds. The antioxidant activities of individual compounds were tested against stable free radical 1,1-Diphenyl-2-picrylhydrazyl radical (DPPH), hydrogen peroxide (H2O2) and FRAP (ferric reducing antioxidant power) and the results were related to the Gallic acid, ascorbic acid and Trolox as standards. The effects revealed that most of the syntheses presented greater activity as an antioxidant than the standards in different concentration range. The distinguished competence inactivating action was observed for compound 2 (86.3±1%) followed by compounds 3 (85±0.5%) using DPHH method. The antioxidant mode of action of the prepared compounds was also investigated.
KEYWORDS:Antioxidant; DPPH; FRAP Hydroxycoumarin H2O2; In Activators; Macromolecules
Download this article as:| Copy the following to cite this article: Duhaidahawi D.L.A, Majedy Y.K.A, Ibraheem H.H, Kadhum A.A.H, Amiery A.A.A. Macro Coumarins as Novel Antioxidants. Orient J Chem 2018;34(5). |
| Copy the following to cite this URL: Duhaidahawi D.L.A, Majedy Y.K.A, Ibraheem H.H, Kadhum A.A.H, Amiery A.A.A. Macro Coumarins as Novel Antioxidants. Orient J Chem 2018;34(5). Available from: http://www.orientjchem.org/?p=50523 |
Introduction
Coumarin is a simple molecule that originates from lactones family. As it possesses a benzopyrone system, it isolates itself from plants. Also, it can undergo total synthesis that is conducted in the laboratory. Furthermore, coumarin can be synthesized from many established chemical reactions. Although coumarins can be obtained from various natural resources, new coumarin derivatives are continuously being discovered and synthesized on regular basis.
Coumarin and coumarin-related compounds have demonstrated much popularity in their used way back for a long time. This is because these compounds have shown significant potential in the production of therapeutic products. They are known for a variety of uses: physiological, antimicrobial,1,2 antioxidant,3,5 anti-inflammatory,6,7 anticoagulant and antitumor8 activities. In recent years, there is much attention in the search for antioxidants from natural, industrial sources. As antioxidants inhibit and scavenge radicals, they play an important role in protecting humans from infections and degenerative diseases. As such antioxidants can be defined as any substrate that significantly delays or prevents oxidation. According to9 in comparison with an oxidizable substrate (lipid, protein, carbohydrate, or DNA) only low concentration of antioxidant is required to delay or prevent numerous diseases conditions such as swelling, carcinogenesis, atherogenesis and also in the aging process as well as after virulent exposure to xenobiotic,10-12
Interest in the discovery of new antioxidant agents has surmounted increased, since the implication of oxidative damages in many pathology cases. The other class of compounds that has caught much attention is polyphenols. This group of molecules comprises flavonoids Flavonoids like flavonols, chalcones, flavanones, flavones, flavan-3-ols in addition to lignins, tannins and tocopherols and phenolic acid.13 However, the flavonoids constitute the largest group of phenolic compounds with a wide range of biochemical and physiological actions like an antioxidant and free radical inactivates characteristics.14 Furthermore, the phenolic molecules can serve as antioxidants by coordinating metal ions, counteracting the generation of radicals and stimulating the antioxidant endogenous system forward.15 Originally, we were utilizing 4-hydroxycoumarin as a preliminary substance and all the integrated coumarins are manifested in Scheme 1.
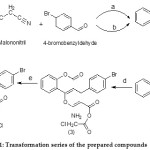 |
Scheme 1: Transformation series of the prepared compounds
|
Chemicals and Environments: a = Tetrabutylammonium bromide (TBAB) / reflux; b = diammonium hydrogen phosphate (DAHP) / reflux; c = hydrolysis; d = benzene/ reflux; e = H2SO4 / reflux.
Experimental Section
General Chemistry
The compounds utilized throughout synthesis were provided by Sigma-Aldrich (Selangor, Malaysia). The infrared ranges were achieved on a Nicolet 6700 FT-IR spectrophotometer (Thermo Nicolet Corp., Madison, WI, USA), and the data are displayed in cm−1. NMR bands were verified manipulating an AVANCE III 600 MHz spectrometer (Bruker, Billerica, MA, USA), DMSO used as a solvent and the data are revealed in δ ppm. CHN analysis was accomplished on an Elemental analyzer Carlo Erba 1108 (Vario El III, SpA, Rodano, Italy).
Chemical Synthesis
Synthesis of 2-amino-4- (4-bromophenyl)-5-oxo-4, 5-dihydropyrano [3, 2-c] chromene-3-carbonitrile (1)
Tetrabutylammonium Bromide (TBAB) as catalytic agent for synthesis of compound (1)
The reactants, 4-Hydroxy-1-benzopyran-2-one (0.162 g; 10 mmol) with 4-bromobenzaldehyde (5 mmol), malononitrile (15 mmol) and Tetra-n-butyl ammonium bromide (10 mol %) were agitated under reflux. Then, the combination was chilled to ambient temperature. The slurry was separated by filtration and desiccated then recrystallized from ethanol.16 Then the compound was characterized by spectroscopic and physical data yields 75%, m.p (223-225)°C.
Diammonium Hydrogen Phosphate (DAHP) as catalytic agent for production of compound(1)
In an aqueous ethanol solution (50% H2O: 50% EtOH) totally 50 ml starting compounds comprising 4-hydroxycoumarin (0.162 g; 10 mmol), 4-bromobenzaldehyde (10 mmol), malononitrile (12 mmol) and ammonium monohydrogen phosphate (26.4 mg, 10 mol %), were agitated for 4 hr. at ambient condition. when the reaction complete, the slurry was separated by filtration and rinsed with ethanol.17 Then the compound was characterized by spectroscopic and physical data. ,yields 70%., m.p ( 225-227°C); 1H-NMR: δ 4.28 (s, 1H, CH), δ 7.11 and δ 7.83 (dd, 2H), δ 7.42-7.83 (m, 1H, C-H aromatic ring), δ8.49 (s, NH2);13CNMR (DMSO): 40, 58.3, 108, 116.0, 125.1, 128.1, 152.1, 160.5, 161.1 and 165.5; FT-IR: 3385.8 cm−1 (NH2), 3189.0 cm−1 (C-H aromatic), 1709.2 cm−1 (C=O, lactone), 2213cm−1 (C≡N), 1638.0 cm−1 (C=C aromatic); CHN analysis: Calcd. for C19H11BrN2O3: C 57.74% H 2.81% N 7.09%, Found: C 57.54% H 2.1% and N 6.91%.
Synthesis of 2-amino-4- (4-bromophenyl)-5-oxo-4, 5-dihydropyrano [3,2-c] chromene-3-carboxylic acid (2)
Aqueous sodium hydroxide solution (1 M, 0.3 ml) was supplemented to compound (1) and the combination was warmed for 5 min at 140 °C. hydrochloric acid (1 M, 0.3 ml) was then added to make solution neutral under these conditions we got compound (2) at 19–20 min. After finishing the reaction, the slurry was separated by filtration and rinsed with ethanol,18 yields: 50%, m.p ( 230-233°C); 1H-NMR: δ 3.93 (s, 1H, CH), δ 7.10and δ 7.81 (dd, 2H), δ 7.32-7.82(m, 4H, C-H aromatic), δ8.5 (s, NH2) , δ 11.0 (s, OH); FT-IR: 3221(NH2), 3307 cm−1 (OH); 3090.1 cm−1 (C-H aromatic), 1652cm−1 (C=O, lactone), 1710 cm−1 (C=O, carboxylic), 1625.3 cm−1 (C=C aromatic); CHN analysis: Calcd. for C19H12BrNO5: C 55.09% H 2.92% N 3.38%, Found :C 54.54% H 2.5% and N 3. 1%.
Synthesis of 2-amino-4- (4-bromophenyl)-5-oxo-4, 5-dihydropyrano [3,2-c] chromene-3-carboxylic 2-chloroacetic anhydride (3)
In 100 ml RBF, (0.02 mol, 9.81 g) of compound (2) in 4ml ethanol was mixed in magnetic agitator for 10 min. Chlor-acetyl chloride (0. 02 mole, 4 equivalent) was supplemented gradually for 1 hr. Reaction combination was maintained on stirring for 24-writhe reaction mixture was chilled, transferred into an ice water (50 ml) including a drop of pyridine and agitated till the oil solidifies. The crude result was filtrated, rinsed with ice-cold water and evaporated. The result was recrystallized from ethanol, yields 45%, m.p (244-246°C); 1H-NMR: δ 2.1 (s, 1H, NH), δ 3.91 (s, 1H, CH), δ 7.2and δ 7.82 (dd, 2H), δ 7.4-7.79 (m, 4H, C-H aromatic ring), δ8.4 (s, NH2); FT-IR: 3433- 3328 cm−1 (NH2), 3055.1cm−1 (C-H aromatic), 1652cm−1 (C=O) 1602.9 cm−1 (C=O, lactone); CHN analysis: Calcd. for C20H14BrClN2O4: C 52.03 % H 3.06 % N 6.07 %, Found: C 51.5% H 2.88% and N 5.81%.
Synthesis of 7-(4-bromophenyl)-10-(chloromethyl)-6H-chromeno [3′, 4’: 5,6] pyrano [2,3-d][1,3] oxazine-6,8(7H)-dione (4)
To a solution of 2 mmol, 0.941g of compound (3) in 50 ml of dry methanol was added concentrated sulphuric acid (80 mmol). The combination was refluxed for (3-4) h. Following cooling; the reaction substance was transferred into an ice-water mixture. The solid particles were collected by percolation, rinsed with water and recrystallized from methanol to obtain compound (4),19 yields 35%, m.p( 217-219°C); 1H-NMR: δ 3.9 (s,2H,CH2Cl), δ 4.4 (s, 1H, CH), δ 7.1and δ 7.8 (dd, 2H), δ 7.4-7.79 (m, 1H??, C-H aromatic ring) ; FT-IR: 3181.6 cm−1 (C-H aromatic), 1670.6, 1612.1 cm−1 (C=O, lactone), 1570.4 cm−1 (C=C aromatic);CHN analysis: Calcd. for C21H11BrClNO5: C 53.36 % H 2.35 % N 2.96 %, Found: C 52.64% H 2.1% and N 1.91%.
Antioxidant activity
(2,2-Diphenyl-1-(2,4,6-trinitrophenyl)hydrazyl) (DPPH) free radical scavenging activity:
Antioxidant properties of synthesized coumarin compounds (1-4) were tested spectrophotometrically utilizing 2,2-diphenyl-1-picrylhydrazyl radical.20,21 At first, 0.1 mL of different strengths of prepared compounds 0.25, 0.5, 0.75 and 1 mg/ml and standard ascorbic acid were combined 1 ml of 0.2 mM DPPH solubilized in CH3OH. The reaction admixture was kept in the darkness for 30 min at 28oC. The control experiment was carried out as above without test samples. The DPPH antioxidant activity was ascertained by determining the absorbance, at 517 nm operating the UV-VIS spectrophotometer. The reduction of DPPH radical in activator was computed relative to the measured absorbance of the control applying the subsequent equation (1):
![]()
Where Ao is the absorbance of the controller reaction, and A1 is the absorbance in the existence of the tests or standards.
H2O2 Scavenging Activity
H2O2 solution (40 mM) was made in buffer phosphate (pH 7.4). Various dilutions (250, 500, 750 and 1000 g ml–1) of the prepared compounds (or ascorbic acid) were supplemented to the H2O2 liquid (0.6 ml, 40 mM). The absorbance of H2O2 at 230 nm was revealed after 10 min in contradiction of a blank solution having phosphate buffer lacking H2O2 22. The H2O2 proportion inactivating activity was estimated applying equation (1).
(FRAP) Ferric Reducing Antioxidant Power Assay
The method suggested by Muss 23 was applied to establish the antioxidant action through FRAP with certain minor adjustments. The FRAP component was made fresh, as in the use of 300 mM acetate buffer, pH 3.6 (3.1 g C2H3NaO2. 3H2O, with 16 ml glacial acetic acid is adjusted up to 1:1 with DW; 10 mM of TPTZ, in 40 mM HCl; and 20 mM FeCl3•6H2O are mixed in a ratio of 10:1:1 followed by incubation at 37°C for 10 minutes prior to the analysis to allow the reagent working time. About 1 ml of FRAP substance was supplemented to 10μl of each compound after the addition of the FRAP reagent, the plates were preserved for 30 minutes at ambient condition and the absorbance of the blue complex (ferrous tripyridyltriazine) was formed from the reaction. All the data were taken in triplicates. The Trolox standard calibration curve was established by plotting the curves of various concentrations in methanol (0.00625 – 0.2 mg/ml) against absorbance (at 595 nm using UV-vis spectrophotometer see Figure 3.26). The calibration equation found for Trolox was r2 = 0.999), where is the optical density at 593 nm and is the concentration (mg/ml) a,b is the constant, r2 is the confidence of coefficient. The findings were reported in mg Trolox equivalents/ g of synthesis compound. The ferric reducing antioxidant power was estimated applying the subsequent method:FRAP = (C x V) / M where FRAP = the ferric reducing antioxidant power, mg/g compound, in TE; C = the concentration of Trolox ascertained from the standardization curve, mg/mL; V= the volume of compound solution, 0.1 mL; M = the weight of synthesis compound, 0.0001 g.
Arithmetical Analysis
Data obtained were presented as mean ± SD and the arithmetical significance of variances was established using one-way assay of variance (ANOVA) by the SPSS 17.0 arithmetical software package. Variances were reflected significant at P<0.05. The significances are represented as mean ± SD (n=3).
Results and Discussion
Antioxidant assay
Antioxidant activity of prepared compounds was achieved manipulating different in vitro tests against 2,2-Diphenyl-1-picrylhydrazyl (DPPH) radical, hydrogen peroxide) inactivating and (FRAP) ferric-reducing antioxidant strength test.
DPPH Antioxidant Activity of Compounds (1-4)
The function of an antioxidant is to eliminate free radicals. One significant assumption of such elimination is by providing hydrogen to FR (free radicals) in its conversion to inactive type.20 The hydrogen addition would eliminate the single electron aspect, which is liable for radical reactivity. Free radicals have been a topic of considerable concern between specialists in the preceding decade. The antioxidant actions of compounds (1-4) were evaluated in vitro utilizing DPPH (1,1-Diphenyl-2-picrylhydrazyl radical radical inactivating methods. Gallic acid was applied as the standard. Donating hydrogen activity, as estimated using DPPH radicals as a hydrogen acceptor, revealed that considerable correlation might be observed linking the concentration of the recently synthesized compound and the inhibition rate. DPPH molecules (1-4) have been proved to diminish the established radical. As indicated by Figure (1) the prepared compounds 1, 2, 3, and 4 possess 61±0.6%, 86.3±1%, 85±0.5%, and 71.2±0.8% DPPH radical-inactivating action. The compounds (1, 2, and 3) contain the N-H group, which enables hydrogen atom transfer (HAT) to the DPPH free radical to provide a resonance-stabilized radical. The scavenging influence enhanced with rising concentrations of samples. In the DPPH technique, the highest antioxidant effect was 90±1% at a strength 1000 μg/mL for compound (2), and the least inactivating power was 26.1±0.5% at 250 μg/mL concentration for compound (1). Figure (2) shows the proposed mechanism for compounds, specifically, 2-amino-4- (4-bromophenyl)-5-oxo-4, 5-dihydro pyrano (3, 2-c) chromene-3-carboxylic acid (2), and 3-amino-2- ((4-bromophenyl)(2-oxo-2H-chromen-3-yl) methyl) acrylic 2-chloro acetic anhydride (3).
Table 1: DPPH antioxidants activity of comp (1-4)
|
Comp NO |
H2O2 inhibition %SD 100µg/ml |
IC50 ±SEM |
|
1 |
61±0.6% |
31±0.2% |
|
2 |
86.3±1.0% |
41.3±1% |
|
3 |
85±0.5% |
41±0.8% |
|
4 |
71.2±0.8% |
26.4±0.5% |
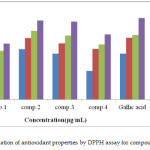 |
Figure 1: Evaluation of antioxidant properties by DPPH assay for compounds (1-4) Click here to View figure |
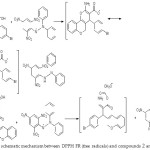 |
Figure 2: The schematic mechanism between DPPH FR (free radicals) and compounds 2 and 3 |
H2O2 Scavenging Capacity
Hydrogen peroxide intact isn’t extremely reactive, but the compound can occasionally be noxious to the cell due to the subsequent hydroxyl radical in cells [24]. Thus, the elimination of H2O2 is very significant for antioxidant protection in the cell. The inactivating ability of prepared compounds on H2O2 equaled with ascorbic acid as a reference compound is shown in Figures 3 the coumarin compounds were able of inactivating H2O2 in a concentration-dependent fashion. Results display that H2O2 scavenging actions of compounds 2and 3 are higher than that of the standard, and the values are 80±1.0%, and 81.5±1.5%, respectively. Compounds 1 and 4show scavenging activity on hydrogen peroxide with lower value compared with ascorbic acid.
Table 2: H2O2 scavenging activity of comp (1-4)
|
Comp NO |
H2O2 inhibition %SD 100µg/ml |
IC50 ±SEM |
|
1 |
60±0.5% |
29±0.5% |
|
2 |
80±1.0% |
39.4±0.3% |
|
3 |
81.5±1.5% |
40±0.6% |
|
4 |
54.2±1.1% |
26.4±0.8% |
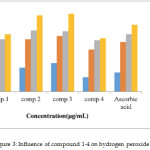 |
Figure 3: Influence of compound 1-4 on hydrogen peroxide |
Ferric Reducing Ability Power (FRAP)
The antioxidant potential of all compounds was ascertained by a FRAP test. The FRAP assessment was revealed as an equivalent of compounds (mg/g of standard antioxidant Trolox). The FRAP test estimated the potency of a compound to reduce the ferric 2,4,6-Tri(2-pyridyl)-s-triazine complex to the stained ferrous complexes compound. FRAP results are acquired by relating the absorbance alteration at 593 nm in assay reaction admixtures with those having ferrous ions in identified concentrations. In general, the reducing properties of these sets of compounds largely rely on the sum and position of hydrogen-donor hydroxyl clusters on the aromatic group of phenolic compounds; in addition, the properties are influenced by other aspects, such as H-donor groups (-NH, -SH) 25, which apply their effect by destroying the free radical series via providing a hydrogen atom.26,27 The FRAP test considers the antioxidants specimen as a reluctant in a redox-associated colorimetric effect 28 following the equation:
![]()
FRAP: [Fe (TPTZ) 2] 2+ (Ferrous tripyridyl triazine cation)
Several synthetic and naturally existing coumarins display possible antioxidant activity. Therefore, the antioxidant influence of coumarin derivatives 1-4 was assessed utilizing FRAP test in contrast to Trolox. The linearity of FRAP (dose–response curve) for conventional solutions is presented in Figure (4). The values of the FRAP test are stated in Table (1), and ferric reducing ability was represented as the concentrations of antioxidant as FRAP value (mg/g). Depending on results, compounds 2 and 3 revealed considerable antioxidant activity compared with Trolox owing to the existence of the NH2 group, which donates a hydrogen atom, in the structure of these compounds. However, the remaining compounds show poor antioxidant power.
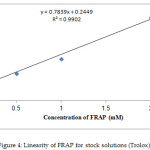 |
Figure 4: Linearity of FRAP for stock solutions (Trolox) |
Table 3: FRAP antioxidant activities of prepared compounds
|
Compound |
FRAP value (mg/ g) |
|
1 |
10.4 |
|
2 |
41.6 |
|
3 |
60.2 |
|
4 |
9.1 |
Conclusion
The preparation and characterization of coumarin derivatives are of considerable interest owing to their potential for various applications based on their biological activity. A series of 4-hydroxy coumarin compounds were successfully synthesized by conventional methods. The recently prepared molecules were described by using elemental analysis (CHN) and spectroscopic methods (IRand 1H and 13C NMR). The scavenging activities of prepared coumarins compounds were verified by utilizing hydrogen peroxide test (H2O2), DPPH antioxidant examines, and FRAP assess. Results indicate that DPPH give the best antioxidant activity observed for compound 2 (86.3±1%) followed by compounds 3 (85±0.5%). The new coumarins hold greater scavenging activity compared with vitamin C, gallic acid, and Trolox as standards.
Conflicts of Interests
Author has none to declare
Acknowledgment
We are grateful to the staff membered at pharmaceutical chemistry department at Kufa University for kind help & support.
References
- Elhafez, O.M.A.; Khrisy, E.E.D.A.M.E.; Badria, F. et al. Synthesis and biological investigations of new thiazolidinone and oxadiazoline coumarin derivatives Arch. Pharm. Res. 2003, 26, 686-696.
CrossRef - Basanagouda, M.; Kulkarni, M.V.; Sharma, D. et al. Synthesis of some new 4-aryloxmethylcoumarins and examination of their antibacterial and antifungal activities J. Chem. Sci. 2009, 121, 485-495.
CrossRef - Parfenov, É.A.; Smirnov, L.D., & Trapkov, V.A. Synthesis and antiulcer activity of copperand zinc-containing coumarin antioxidants. Pharm. Chem. J. 2005, 30, 445-447;
- Darwish, E.S.; Fattah, A.M.; Abdel Attaby, F.A.; Al-Shayea, O.N. Synthesis and Antimicrobial Evaluation of Some Novel Thiazole, Pyridone, Pyrazole, Chromene, Hydrazone Derivatives Bearing a Biologically Active Sulfonamide Moiety.Int. J. Mol. Sci. 2014, 15, 1237-1254.
CrossRef - Al-Amiery, A. A.; Al-Majedy, Y. K.; Kadhum, A. A. H., & Mohamad, A. B. Novel macromolecules derived from coumarin: synthesis and antioxidant activity. Sci. Rep., 2015, 5, 11825.
CrossRef - Aiyelabola, T.; Akinkunmi, E.; Obuotor, E.; Olawuni, I.; Isabirye, D. & Jordaan, J. Synthesis Characterization and Biological Activities of Coordination Compounds of 4-Hydroxy-3-nitro-2H-chromen-2-one and Its Aminoethanoic Acid and Pyrrolidine-2-carboxylic Acid Mixed Ligand Complexes. Bioinorg. Chem. Appl. 2017,2017, 6426747.
- Shaimaa A. M.; Abdelbasset A. F.; Magda N.A. N.; Atif S. T. Synthesis, molecular modeling and anticancer activity of new coumarin containing compounds. Saudi Pharm. J., 2017,25, 873-883.
CrossRef - Marchenko, M.M.; Kopyl’chuk, G.P.; Shmarakov, I.A. et al. Synthesis and antitumor activity of 5-(5′,6′-benzocoumaro-3′-yl)methylaminouracil hydrobromide and its liposomal medicinal form. Pharm. Chem. J.2006, 40, 296. FereIdoon S. Antioxidants for food preservations. Woodhead Publishing elseiver, UK 2015, number 276, pp 238-241.
- Miri, R.; Saadati, H.; Ardi, P.; Firuzi, O. Alterations in oxidative stress biomarkers associated with mild hyperlipidemia and smoking. Food Chem. Toxicol. 2012,50, 920-926.
CrossRef - Grešner, P., Świercz, R., Król, M.B. et al. Does the Low-level occupational exposure to volatile organic compounds alter the seasonal variation of selected markers of oxidative stress? A case–control study in nail technicians. J Occup Med Toxicol. 2016, 11, 36.
CrossRef - Sies, H., Berndt, H. C., and Jones, D. P. Oxidative Stress. (2017) Oxidative stress. Annu. Rev. Biochem. 86, 715–748.
CrossRef - Mehrdad, A.; Javad, K.; Mahdi, K.; Farhad, A.; Mahnaz, A. Comparison of total phenolic and antioxidant activity of different Mentha spicata and M. longifolia accessions. Ann. Agric. Sci., 2016, 61, 175-179.
CrossRef - Al-Azzawie, H.F.; Alhamdani, M.S. Hypoglycemic and antioxidant effect of oleuropein in alloxan-diabetic rabbits.Life Sci. 2006,78,1371-7.
CrossRef - Khurana, J. M.; Kumar, S. Tetrabutylammonium bromide (TBAB): a neutral and efficient catalyst for the synthesis of biscoumarin and 3, 4-dihydropyrano [c] chromene derivatives in water and solvent-free conditions. Tetrahed. Lett. 2009,50, 4125-4127.
CrossRef - Divsalar, N.; Monadi, N.; Tajbaksh, M. Preparation and Characterization of a Molybdenum(VI) Schiff Base Complex as Magnetic Nanocatalyst for Synthesis of 2-Amino-4H-benzo[h]chromenes. J. Nanostruct. 2016, 6, 312-321.
- Reid, A.E.; Kim, S.W.; Seiner, B.; Fowler, F.W.; Hooker, J.; Ferrieri, R.; Babst, B. Fowler, J.S. Radiosynthesis of C-11 labeled auxin (3-indolyl[1- 11C]acetic acid) and its derivatives from gramine. J. Label Compd. Radiopharm 2011, 54 433–437.
CrossRef - Serban, G. 5-Arylamino-1, 3, 4-thiadiazol-2-yl acetic acid esters as intermediates for the synthesis of new bisheterocyclic compounds. Farmacia 2015,63, 146-149.
- Kadhum, A.A.H; Al-Amiery, A.A.; Musa, A.Y.; Mohamad, A.B. The Antioxidant Activity of New Coumarin Derivatives. Int. J. Mol. Sci.. 2011;12(9):5747-5761.
CrossRef - Abdul Amir, H. K.; Ahmed, A. A.; Mukaram, S.; AbuBakar, M. Synthesis, structure elucidation and DFT studies of new thiadiazoles. Int. J. Phys. Sci.2011, 29, 6692 – 6697.
CrossRef - Ahmed, A.A.; Abdul Amir, H. K.; Hasan, R.O.; Abu Bakar, M. Synthesis and Antioxidant Activities of Novel 5-Chlorocurcumin, Complemented by Semiempirical Calculations. Bioinorg. Chem. Appl. 2013; 2013: 354982.
- Musa, K. H.; Abdullah, A.; Jusoh, K.; Subramaniam, V. Antioxidant Activity of Pink-Flesh Guava (Psidium guajava L.): Effect of Extraction Techniques and Solvents. Food Anal. Methods, 2011 4, 100-107.
- Goufo, P.; Trindade, H. Rice antioxidants: phenolic acids, flavonoids, anthocyanins, proanthocyanidins, tocopherols, tocotrienols, γ-oryzanol, and phytic acid. Food Sci. Nutr. 2014,2,75-104.
CrossRef - Prakash J.D.; Prakash J.; Lakshmi A. J.;Fatih Y. Antioxidant properties of fresh and processed Citrus aurantium fruit. Cogent Food Agric. 2014, 2, 2016.
- Prasad, K.N.; Chew, L.Y.; Khoo, H.E.; Kong, K.W.; Azlan, A. Ismail A. Antioxidant Capacities of Peel, Pulp, and Seed Fractions of Canarium odontophyllum Miq. Fruit. J. Biomed. Biotechnol. 2010, 2010, 871379.
- Kumar, S.; Sharma, S., Vasudeva N. Review on antioxidants and evaluation procedures. Chin. J. Integr. Med.2017,1-7.
CrossRef - Yehye, W.A.; Rahman, N.A.; Ariffin, A.; Abd Hamid, S.B.; Alhadi, A.A.; Kadir, F.A.; Yaeghoobi, M. Understanding the chemistry behind the antioxidant activities of butylated hydroxytoluene (BHT): a review. Eur. J. Med. Chem. 2015,101,295-312.
CrossRef - Comparison of ABTS, DPPH, FRAP, and ORAC assays for estimating antioxidant activity from guava fruit extracts
- Kriengsak, T.; Unaroj, B.; Kevin, C.; Luis, C-Z.; David, H. B. Comparison of ABTS, DPPH, FRAP, and ORAC assays for estimating antioxidant activity from guava fruit extracts. J.Food Compost. Anal. 2006, 19, 669-675.
CrossRef

This work is licensed under a Creative Commons Attribution 4.0 International License.









