Oxidative Desulfurization of Hydrotreated Gas Oil using Fe2O3 and Pd Loaded over Activated Carbon as Catalysts
Jalil R. Ugal1, Rawnaq B. Jima'a2 , Wessal Metaab Khamis Al-Jubori3, Bayader Fadhel Abbas3 and Nedhal Metaab Al-Jubori 2
, Wessal Metaab Khamis Al-Jubori3, Bayader Fadhel Abbas3 and Nedhal Metaab Al-Jubori 2
1Baghdad University, College of Sciences for women, Chemistry Department, Baghdad, Iraq.
2Diyala University, College of Sciences, Chemistry Department, Diyala, Iraq.
3Al-Mustansiriyah University, College of Sciences, Chemistry Department, Baghdad, Iraq.
Corresponding Author E-mail: rawnaq@sciences.uodiyala.edu.iq
DOI : http://dx.doi.org/10.13005/ojc/340261
Article Received on : January 11, 2018
Article Accepted on : February 28, 2018
This paper discusses preparation of catalysts Fe2O3/ Ac and Pd/ Ac by precipitation method according to the ratio cat. :Ac (20:80) and evaluates their catalytic activity to remove sulfur compounds from hydrotreated gas oil. The chemical structure of the prepared catalysts were examined by X- ray diffraction (XRD) analysis, atomic absorption spectroscopy (AAS), surface area and pore volume, and thermal gravimetric analysis (TGA). X-ray fluorescence spectrometer used to determine the content of sulfur in gas oil before and after the treatment. The catalysts in this study showed an efficient removal of sulfur compounds from light gas oil reaches to more than 60%
KEYWORDS:Oxidative desulfurization; Hydrotreated gas oil; Ferric oxide over active carbon catalyst; Palladium over active carbon catalyst
Download this article as:| Copy the following to cite this article: Ugal J. R, Jima'a R. B, Al-Jubori W. M. K, Abbas B. F, Al-Jubori N. M. Oxidative Desulfurization of Hydrotreated Gas Oil using Fe2O3 and Pd Loaded over Activated Carbon as Catalysts. Orient J Chem 2018;34(2). |
| Copy the following to cite this URL: Ugal J. R, Jima'a R. B, Al-Jubori W. M. K, Abbas B. F, Al-Jubori N. M. Oxidative Desulfurization of Hydrotreated Gas Oil using Fe2O3 and Pd Loaded over Activated Carbon as Catalysts. Orient J Chem 2018;34(2). Available from: http://www.orientjchem.org/?p=44955 |
Introduction
Refining factories play an important rule to transfer coat oil to more valuable products such as liquid petroleum gas, jet fuel, gasoline, and diesel by using various techniques like distillation, extraction, reforming, hydrogenation, and cracking1.
Millions of barrels of fuels are consumed daily in the worldwide road transportation therefore; increasing attention is being focus to the composition of fuel processing. The big challenge that faces the refineries is the harmful sulfur oxides which liberate to the environment from the combustion of petroleum fuel that contained high sulfur 2,3. Sulfur exists in hydrotreated petroleum as hydrogen sulfide, disulfides, organic sulfides, benzothiophene, dibenzothiophene, and their alkylated derivatives 4,5. These compounds are unwanted and detrimental pollutants that converted to gaseous sulfur oxides (SOX) upon fuel combustion which causes an acidic rain and the contamination of the atmosphere 6.
Oxidative desulfurization (ODS) is a very effective and considers a promising method for removing the dibenzothiophene derivatives from liquid fuel under moderate conditions, as low temperature, pressure and the cost of operation is less compared to HDS 7,10.The OSD method is a kind of technology using oxidants to oxidize organic sulfur in two steps: the first step; oxidation of the sulfur atoms to strong polarity species and these polar products can be effectively separated by liquid extraction in the final step. In OSD the sulfur containing compounds in fuels are oxidized using different oxidizing agents such as nitric acid, nitrogen dioxide, organic hydro peroxides, hydrogen peroxide, peroxy acids, and ozone in the presence of a catalyst to produce sulfoxides and sulfones compounds respectively 9,14. By that means, the oxidation of sulfides to sulfoxides and sulfones is generally accomplished by reaction with peroxy acid which generated by the reaction between peroxide and appropriate carboxylic acid. These oxidized molecules due to the strong polarity of them compared to the respective sulfides and the liquid extraction can be more efficient way to remove selectively the oxidized sulfur compounds from oil layer 15,16.
This work contributes to a growing interest to produce low or no sulfur containing compounds in gas oil. The oxidation of gas oil by hydrogen peroxide using the catalysts ferric oxide Fe2O3/ Ac, palladium Pd/ Ac prepared in this work.
Experimental
Chemicals and Reagents
The analytical grade chemicals and reagents used in this study are ferric nitrate Fe(NO3)3.9H2O, sodium hydroxide, and hydrochloric acid purchased from Fluka, palladium chloride PdCl2 and charcoal from Sigma company. Also, barium chloride BaCl2, sodium bicarbonate NaHCO3, sodium carbonate Na2CO3, and acetonitrile CH3CN were supplied from BDH. In addition, the oxidizing agent for ODS procedure is hydrogen peroxide H2O2 (30%w/w) and glacial acetic acid C2H4O2 16N, nitric acid HNO3, 12N, were equipped from Scharlau. While, the hydrotreated gas oil used in this study provided from Midland Refineries Company in Iraq which analyzed using X-ray fluorescence to determine total sulfur content (wt (%)) equal to 1.477 (1477ppm), API-Gravity= 31.9, and specific gravity= 0.85, distilled water used prior the experiments.
Preparation of Palladium, and Ferric Oxide Loaded Over Activated Carbon
The catalysts in this study were prepared according to the ratio cat.:Ac (20:80). Fe2O3/ Ac catalyst was prepared as reported by Hussein H. A. 17 as follow: a calculated weight of Fe(NO3)3.6H2O was dissolved individually in 40ml distilled water. The precipitation of metal achieved by adding 1.0M NaHCO3 to the solution gradually until pH of the solution reached to 8-9 and the content was continuously stirred at 60C° for 3h. After that, the mixture was filtered through a Buchner funnel in vacuum and washed using distilled water to get rid of sodium ions. To make sure that the precipitate was free of sodium ions few drops of 0.1M BaCl2 was added to the filtrate as filtrate turbidity means the presence of sodium ions so continued in the washing with water until it is free from sodium ions. The precipitate cake was dried in an oven at 120°C for 24h to remove water.
The required weight of active carbon to achieve the ratio of cat.: Ac (20: 80) dried at 120°C for 24h and then wetted with 10ml of distilled water. The calculated weight of the catalyst prepared earlier dissolved individually in (5ml concentrated HCl: 40ml distilled water) and mixed with continuous stirring with the calculated weight of active carbon and the mixture left for 24h at room temperature to allow the most of metal solution to be loaded on the activated carbon. The dried catalyst is calcinated for 2h at 400°C in muffle furnace and the purpose of this step is to load the metal salts on the active carbon and converted them to their corresponding metal oxides leading to deposit of active metal oxide over the support. Also, in the calcination step generate strong interaction between metal oxide and the support (Activated carbon). While, the preparation of Pd/C include dissolving of a calculated amount of PdCl2 in hydrochloric acid (PdCl2 doesn’t dissolve in water).
The impregnation step of Pd on carbon has been done by adding (10ml) of formaldehyde CH2O to PdCl2 solution to reduce palladium ions Pd+2 to Pd as metal precipitated on active carbon. After that, neutralization the solution by adding sodium hydroxide with continuous stirring and remove chlorides ions by washing the precipitate with distilled water. The drying step was important to get rid of water and moisture at 120°C. Before the impregnation of Fe2O3 and Pd on activated carbon, charcoal was mixed with nitric acid HNO3 at room temperature for 24h to increase reactivity and porosity of carbon. After 24h, the resultant active carbon filtered, washed many times with distilled water to remove the residual of acid in its pores and then dried at 120°C for 2h.
Procedure of Oxidative Desulfurization Experiments
The ODS experiments were performed in a water bath at 40°C with 5ml of glacial acetic acid, 10ml of H2O2 mixed with 100ml of gas oil and 3gm of the catalyst in a three necks round bottom flask with. The mixture was stirred for 3h using a mechanical stirrer at 1000rpm, during the stirring process a solution of 2gm Na2CO3 dissolved in 20ml of distilled water was added drop by drop to the mixture. After the oxidation was finished the mixture attained phase separation, the oxidized sulfur was extracted with acetonitrile at room temperature. The acetonitrile and gas oil ratio was 1:1 by volume then the gas oil phase was separated and analyzed using X-ray fluorescence spectrometer ARL 8410 (ASTM D4294 method). The removal efficiency of sulfur compounds is calculated using the following equation:
Efficiency of desulfurization= (s° – s1/ s°)*100
Where S0 is the initial S-content and S1 is the final S-content after (ODS). The results indicate that the catalyst is efficient to remove sulfur from gas oil with the catalyst
Instruments used in the Structural Characterization
Crystalline structure of the catalysts was examined by X-ray diffraction (Shimadzua-XRD-6000, Japan) equipped with Cu Ka radiation (ƛ= 1.5406A°) with a scanning speed 8° per min., 40Kv and 30mA, 2θ from 10° to 80°. Atomic absorption spectroscopy type (Shimadzu Europe AA-6300) used to determine the percentage of elements Pd and Fe in the prepared catalysts Pd/Ac and Fe2O3/Ac respectively. HORIBA/SA9600 series was used to measure the surface area and pore volume.
Also, thermal gravimetric analysis was performed to determine the thermal stability of oxide was performed by TGA-4000 instrument from Perkin-Elmer/Holland.
X-ray fluorescence analyzer (Lab X-300) used to calculate the content of sulfur in gas oil before and after treatment.
Results and discussion
X-ray Diffraction
X-ray diffraction (XRD) gives crystallographic and amorphous information of the prepared catalysts loaded over an active carbon. Fig. 1 and 2 show the XRD patterns for the supported catalysts prepared in this work. The diffractogram of the calcined Fe2O3/ C at 400C° shows the main diffraction peaks at 33.34°, 35.68°, 49.44°, 54.12°, and 62.54° which are related to Iron oxide in the form of hematite Fe2O3 and magnetite Fe3O4. It is assumed that the Iron oxide in the form of magnetite particles are formed mainly inside the support pores in the calcination step after the impregnation of ferric nitrate over the support. The formation of magnetite is attributed to the reduction some of Fe+3 to Fe+2 by carbon particles in the presence of high temperature (calcination step). Reduction agent in the carbon is the carbon monoxide CO resulting from burning of the activated carbon, carbon monoxide dose not has the ability to access to inside of the catalyst so, magnetite is formed on the surface. Therefore, the final result particles formed are hematite the central part surrounded by magnetite 18.
The XRD investigation for Pd/ C reveals three diffraction peaks at 40.32°, 46.6° and 68.3° related to the Pd face-centered cubic crystal structure as in Fig. 2. Also, there is a characteristic values for 2θ at 24.25° and 25.13° attributed to carbon particles which supported Fe2O3 and Pd respectively. These results match with the earlier studies with simple variation due to the difference in the calcination temperature 19,21.
The peaks of the patterns of Fe2O3/C and Pd/C catalysts are in good agreement with those of the standard XRD JCPDS data: PDF number 39-1346 and PDF-number 046-1043 respectively.
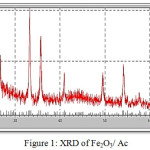 |
Figure 1: XRD of Fe2O3/ Ac
|
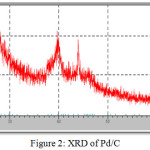 |
Figure 2: XRD of Pd/C
|
Atomic Absorption Spectroscopy
Atomic absorption spectroscopy technique utilizes to determine the percentage of metal ions presented in the catalysts. Table (1) reveals that the prepared catalysts containing on 1.1149% and 5.8708% of Pd and Fe in their composition respectively.
Table 1: Percent of metal and metal oxide in the prepared catalyst.
| Catalyst | Metal (%) | Metal oxide (%) | Weight of carbon (gm) |
| Fe2O3/ Ac | 5.8708 | 16.79 | 16 |
| Pd/ Ac | 1.1149 | – | 16 |
Determination of surface area and pore volume
Also, determination of the surface area and pore volume of the catalysts are fundamental aspect, since such properties can influence the catalytic activity and mass transport of reactants and products22. The data in Table (2) exhibit surface area and pore volume of Fe2O3/C and Pd/C.
Table 2: Surface area and pore volume of Fe2O3 and Pd loaded over carbon
| Catalyst | Surface Area m2/gm | Pore Volume cm3/gm |
| Fe2O3/ Ac | 44.260 | 0.233 |
| Pd/ Ac | 70.179 | 0.200 |
Thermal Analysis
Thermal gravimetric analysis studied for Fe2O3/C as in Fig. 3. The TGA curve showed, there was losing of weight at two points; first one at 271C° which was around 8.197%. After TGA calculation, the compound suffered losing in weight around 13.4 which could be equivalent to the notional value. The second point occurred at 595.5 and has the percent 13.351% and practical value was 2.57 which is parallel with the theoretical value. The weight losing of ferric oxide at the two points have small value. This can be attributed to loss of water molecules that oxide absorbed it from the surrounding 25. The second prospect for losing in weight can be represented to transform the oxide from γ formula to the more stable formula (α) especially that α formula of ferric oxide is prepared at 700 ᵒC and while in this work (α) formula was prepared at 400C°11.
 |
Figure 3: TGA curve of Fe2O3/C
|
Oxidative desulfurization of gas oil using Pd/ C & Fe2O3/ C
Oxidative desulfurization based on the use of gas oil was conducted as mentioned in literature 20 using hydrotreated gas oil (sulfur content 1477ppm, 100ml) with 3gm of the prepared catalyst mixed with 30% H2O2 as an oxidizing agent followed by adding 5ml of CH3COOH as a co-catalyst. The mixture refluxed for 3h at 40°C using a mechanical stirrer at 1000rpm and while stirring 2gm of Na2CO3 dissolved in 20 ml of DW added to the mixture. At the end of reaction, the mixture left to settle for 30min to attain phase separation. The biphasic mixture was separated by extraction with acetonitrile and 2% barium chloride. Clearly a white precipitate of barium sulfate BaSO4 was formed in the aqueous layer.
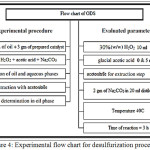 |
Figure 4: Experimental flow chart for desulfurization procedure
|
Results of the oxidation of gas oil containing on sulfur compounds in the presence and absence of acetic acid as a co-catalyst are shown in Table.(3). It can be seen that the OXD process is more effective in the presence of the acetic acid than without it. In the same table the effect of hydrogen peroxide and Na2CO3 on the OXD process also studied. The removal of sulfur was 71.20% and 66.0% for Fe2O3/Ac and Pd/Ac respectively. H2O2 is considered as a green reagent which is oxidizing organic sulfur compounds to the corresponding sulfones at ambient temperature.
Table 3: Efficiency removal of sulfur compounds in the presence and absence of CH3COOH and different amount of H2O2 and Na2CO3 at 40C° and 3h the time of reaction.
| No. Exp. | Catalyst | Vol. of CH3COOH (ml) | Vol. of H2O2 (ml) |
Amounts of Na2CO3 (gm) |
Removal Efficiency (%) |
| 1 | Fe2O3/ Ac | 0.0 | 10 | 0.0 | 12.10 |
| 2 | Pd/ Ac | 0.0 | 10 | 0.0 | 10.40 |
| 3 | Fe2O3/ Ac | 5.0 | 10 | 2 | 71.2 |
| 4 | Pd/ Ac | 5.0 | 10 | 2 | 66.0 |
| 5 | Fe2O3/ Ac | 5.0 | 15 | 2 | 17.03 |
| 6 | Pd/ Ac | 5.0 | 15 | 2 | 13.24 |
| 7 | Fe2O3/ Ac | 0.0 | 10 | 0.0 | 16.10 |
| 8 | Pd/ Ac | 0.0 | 10 | 0.0 | 18.24 |
The results in Table (3) indicates that the higher removal efficiency of sulfur from hydrotreated gas oil was achieved when using 10ml of H2O2 as oxidizing agent, 5ml of CH3COOH as co-catalyst, and 2gm of Na2CO3.
Mechanism of the oxidation reaction
A reasonable mechanism of oxidative desulfurization process can be suggested as an active catalyst species can be formed by the nucleophilic attack of hydrogen peroxide on metal atoms of the catalyst. Electrons are evacuated from the peroxyl ions, because of that increasing the electrophilic character of the peroxidic oxygen. The ODS moves by nucleophilic attack of an active catalyst (oxidants) and convert sulfur containing compounds in fuels to form sulfones which are much more polar species. Fig.5 represents the mechanism of ODS in liquid phase 20, 25.
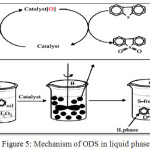 |
Figure 5: Mechanism of ODS in liquid phase
|
Conclusion
In this study, Fe2O3/ Ac and Pd/ Ac were prepared by precipitation method in ratio Cat.:Ac equal to 20:80 and used as catalysts for the oxidation of sulfur compounds in gas oil. It was found that the catalytic activity at selected parameters: 100 ml of gas oil mixed with 3gm of the catalyst in the presence of H2O2 as oxidizing agent and CH3COOH and Na2CO3 as a co-catalyst. Results obtained in this study for gas oil samples showed that the use of the prepared catalysts achieved an efficiently removal of sulfur in gas oil using eco friendly reagents reached to 71.20% and 66.0% for Fe2O3/ Ac and Pd/Ac respectively.
Acknowledgement
The authors are grateful to the Head department of Chemistry, College of Sciences, Diyala University and the Head of Chemical Engineering, College of Engineering, Diyala University for providing the necessary support and technical assistant to accomplish this study.
References
- Katzer, J. R.; Ramage, M. P.; and Sapre, A. V. Chem. Eng. Prog., 2000, 6, 41–51.
- Song, C.; Ma, X. Appl. Catal. B., 2003, 207–238.
CrossRef - Lam, V.; Li, G.; Song, C.; Chen, J.; Fairbridge, C.;Hui, R.; Zhang, J. Fuel Process. Technol., 2012, 98, 30–38.
CrossRef - Speight, JG. Handbook of petroleum product analysis. New Jersey: John Wiley and Sons; 2002.
- Hagen, J. Industrial catalysis— a practical approach. 2nd ed. Germany: Wiley-VCH Verlag GmbH & Co, KGaA.; 2006.
- Collins, FM.; Lucy AR.; Sharp, C. J Mol Catal A: Chem., 1997, 117, 397-403.
CrossRef - Dishun, Z.; Fengxia, S.; Erpeng, Z.; Yan, L.; A Review of desulfurization of light oil based on selective oxidation. China: College of Chemistry and Pharmaceutical Engineering; 2003 October.
- Breysse, M; Diega-Mariadassou, G.; Pessayre, S.; Geantet, G.; Vrinet, M.; Lemaire, M. Catal. Today., 2003, 84, 129–138.
CrossRef - Tam, P.S.; Kittrell, J.R.; Eldridge, J.W. Ind. Eng. Chem. Res., 1990, 29, 321-324.
CrossRef - Chica, A.; Corma, A.; Dómine, M.E. J. Catal., 2006, 242, 299-308.
CrossRef - Karas, L.J.; Grey, R.A.; Lynch, M.W. Desulfurization process. US Patent 2008047875, 2008.
- Zannikos, F.; Lois, E.; Stournas, S. Fuel Process. Technol., 1995, 42, 35-45.
CrossRef - Otsuki, S.; Nonaka, T.; Takashima, N.; Qian, W.; Ishihara, A.; Imai, T.; Kabe, T. Energy Fuels., 2000, 14, 1232-1239.
CrossRef - Otsuki, S.; Nonaka, T.; Qian, W.; Ishihara, A.; Kabe, T. J. Jpn. Pet. Inst., 1999, 42(5), 315-320.
CrossRef - Ukkirapandian, V.; Sadasivam, V.; Sivasankar, B.; Pet Sci Technol., 2008, 73, 423–35.
CrossRef - De Filippis, P.; Scarcella, M. Energy Fuels., 2003, 17, 1452–5.
CrossRef - Hussein, A. H. Preparation and characterization of bimetallic naked and alumina supported catalyst for desulfurization of gas oil [Thesis]. University of Baghdad; 2014 May, 101p.
- Lima, S. B.; Borges, S. M. S.; do C. Rangel, M.; Marchetti, S. G. J. Braz. Chem. Soc., 2013, 24(2), 344-354.
CrossRef - Ivashchenko, N. A.; Gac, W.; Tertykh, V. A.; Yanishpolskii, V. V.; Khainakov, S. A.; Dikhtiarenko, A. V.; Pasieczna-Patkowska, S.; Zawadzki, W. World Journal of Nano Science and Engineering., 2012, 2, 117-125.
- Ugal, J. R.; Hussein, A. H. Hussein. Baghdad Science Journal., 2016, 13, 75-83.
CrossRef - L´opez-Romero, S.; Morales Leal, F. REVISTA MEXICANA DE FI´SICA 2011, 57(3), 236-240.
- Ertl, G.; Knozinger, H.; Weitkamp, J. Germany: Handbook of Heterogeneous Catalysis. Wiley-VCH: Weinheim., 1997, 2, 2497.
- Ritu, M. Journal of Applied Chemistry., 2013, 4(6), 41-46.
- Phu, N. D.; Duc-The Ngo, Ph.;, Huy, H. L.; Nguyen, H. H. Journal of Physics D: Applied Physics., 2011, 44(34), 345002.
CrossRef - Trakarnpruk, W.; Rujiraworawut, K. J. Fuel Processing Technology., 2009, 90, 411-414.
CrossRef

This work is licensed under a Creative Commons Attribution 4.0 International License.









