A Ferro-alloy, Calcium Carbide and Zinc Sublimates, Production from the Achisay Deposit Ore (Complex tests)
Viktor Mikhaylovich Shevko , Gulnara Ergeshovna Karataeva, Alexandra Dmitrievna Badikova, Mustafa Azatovich Tuleev and Daniel Daniarovich Amanov
, Gulnara Ergeshovna Karataeva, Alexandra Dmitrievna Badikova, Mustafa Azatovich Tuleev and Daniel Daniarovich Amanov
South Kazakhstan State University named after M.Auezov, Kazakhstan,160012, Shymkent, Tauke Khan avenue, 5.
Corresponding Author E-mail: v.m.shevko@mail.ru
DOI : http://dx.doi.org/10.13005/ojc/340269
Article Received on : October 17, 2017
Article Accepted on : January 05, 2018
The present article contains the research results of electrosmelting the Achisay zinc oxide ore with extraction of silicon, calcium, iron, zinc and lead in a commodity output. It is found, that in a system of the Achisayore – carbon the calcium carbide formation begins at 1800°С, silicon and iron silicides – at 1500°С. Zinc completely passes in a gas phase at 1800°С, lead at 2300°С. At the electrosmelting the Achisayore (10,1% of Zn, 0,4% of Pb, 17,1% of Fe, 12,0% of Ca, 2,7% of Si) in the presence of coke and quartzite the ferroalloy with the Si content of 25,7-27,1% and the calcium carbide with capacity of 228 l/kg have been obtained. The calcium carbide capacity increases to 275 l/kg at the addition of lime in the charge (in this case the sublimates contain 44,34% of Zn). The suggested method of the zinc oxide ore processing allows to increase a level of complex processing the ore in 2,53 times.
KEYWORDS:Ferroalloy; Calcium Carbide; Zinc Sublimates; Electrofusion; Zinc-Containing Oxide Ore
Download this article as:| Copy the following to cite this article: Shevko V. M, Karataeva G. E, Badikova A. D, Tuleev M. A, Amanov D. D. A Ferro-alloy, Calcium Carbide and Zinc Sublimates, Production from the Achisay Deposit Ore (Complex tests). Orient J Chem 2018;34(2). |
| Copy the following to cite this URL: Shevko V. M, Karataeva G. E, Badikova A. D, Tuleev M. A, Amanov D. D. A Ferro-alloy, Calcium Carbide and Zinc Sublimates, Production from the Achisay Deposit Ore (Complex tests). Orient J Chem 2018;34(2). Available from: http://www.orientjchem.org/?p=44154 |
Introduction
Kazakhstan owns some deposits containing zinc in an oxide form. These are Achisay, Shaymerden, Shalkiya and Zhayrem deposits [1, 2, 3]. Zinc reserves in these deposits make 1,5-1,6 million tonnes. Similar ores are also available in the USA (Mississippi), Bulgaria (Sedmochislentsy), and Poland (Bytom, Olkush) [4]. A basic way of processing these ores is pyrometallurgy (waelzprocess,distillation in retorts, shaft and electric furnaces) [5]. This method allows extracting in sublimates to 88% of Zn and 90% of Pb. However the pyrometallurgical methods have a number of essential disadvantages; for this reason, despite a number of improvements [6, 7, 8], they cannot be considered as technologies of the future. So, distillation in shaft electric furnaces is characterized by high (triple) coke surplus and high zinc content in the waste (90%). During a Sterling process (electrosmelting) the dump slag is formed, which contains Fe, Si, Ca, Al and partly Zn, that leads to loss of these valuable components. At processing in accordance with the JSP method (distillation in shaft furnaces) Fe, Si, Ca, Al are also lost with a slag, and Zn content in the slag reaches 7%. A Waelz process, which time is 2-2,5 hours, demands a considerable quantity of coke (45-55% from the ore weight); in addition during the process the waste clinker is formed (to 90% from the ore weight) with which Fe, Si, Ca and to 30% of the coke are lost. Known methods of zinc oxide ore extraction have mainly one purpose – simultaneous extraction of zinc and other accompanying nonferrous metals [9, 10, 11, 12]. In this case a dump cake is formed, which is not processed.
On the basis of this fact the problem emergent at the zinc oxide ore processing is increase of their complex processing level with using not only nonferrous metals, but also other components (Si, Fe, and Ca). With that end in view we suggest the electrothermal method providing for combination of obtaining siliceous ferroalloys, calcium carbide and zinc sublimates in one unit – an electric furnace [13, 14, 15]; the process realizes according to the reactions:
ZnO +SiO2 + CaO + FeO +7C = FeSi +CaC2 + Zn + 5CO (1)
ZnO +SiO2 + CaO + 6Fe +C = FeSi + CaC2 + Zn + 4CO (2)
From the thermodynamic point of view these reactions are possible at 1600,6K and 1724,3K, respectively (the present calculation has been performed by us by means of a HSC-5.1 software package [16]).
Earlier we had carried out thermodynamic modelling of the process, studied kinetics and optimum technological parameters of joint production of a ferroalloy, calcium carbide and zinc sublimates from the Achisay zinc oxide ores [17]. The given article contains the results of realization of the developed technology as complex laboratory researches.
Raw Materials
There are two kinds of zinc ores on the Achisay deposit: rich ores (Zn>10%), being in a mine and poor ones(Zn≤5%), which are on a surface as a waste ore. Figure 1 is represented the results of electron microscopic analysis of these two kinds of the Achisayores which show that the rich ores contain 12,66-19,2% of zinc, and the poor – 1,0-3,54% of zinc.
 |
Figure 1: The Achisay ore scanning electron microscopy |
For performance of the research we have used a raw mixture including the rich and poor ores (50:50). The ore mixture composition is 10,1% of Zn, 0,4% of Pb, 17,1% of Fe, 12,0% of Ca, 0,1% of Mn, 0,1% of K, 2,7% of Si, 1,1% of Na, 0,5% of Al, 3,8% of Mg, 40,7% of O, 11,8% of C. Coke, quartzite and lime compositions are represented in table 1.
Table 1: Raw material composition
| Raw material |
Content, % |
||||||||
|
SiO2 |
CaO |
MgO |
Fe2O3 |
Al2O3 |
S |
C |
H2O |
other |
|
| Coke |
4,9 |
1,5 |
0,4 |
2,2 |
1,8 |
0,8 |
86 |
1,1 |
1,3 |
| Lime |
2,0 |
93,4 |
1,1 |
0,9 |
1,5 |
0,1 |
0,5(СО2) |
0,3 |
0,2 |
| Quartzite |
97,0 |
0,7 |
0,3 |
0,8 |
0,7 |
– |
– |
0,2 |
0,3 |
The Research Technique
Before the investigation the initial materials had been dried at 120°С and grinded in a jaw crusher. For making a charge the raw materials of 0,5-1,5 mm fraction have been applied.
The electrosmelting has been realized in a monoelectrode arc electric furnace with a carbon-graphite bottom and a chrome-magnesite lining. The furnace stack height is 25 cm. The furnace bath in horizontal section has cuts of 20х20 cm. The bottom is located with a bias of 10 degrees to the notch with d=3 cm. The furnace cover is made from the chrome-magnesite bricks put into a metal frame. The cover has an aperture for an electrode and a bar for opening of the cover at loading a charge. One bus from the transformer is connected to (through a wedge ring) to a graphite electrode with a diameter of 7 cm, and the second with copper studs connected to the bottom. Vertical movement of the electrode was carried out by means of a screw movement mechanism. The furnace transformer has a current range from 0 to 1500А and a pressure interval from 0 to 56V. Continuous current and pressure adjustment is realized by means of a territor regulator, and also by the electrode movement. The control of electric parameters is performed with use of the amperemeter and the voltmeter on the low side of the transformer (TENGEN 4216 GB/T7676-1998 and CHNT 4216, respectively, with an error of 1,5% (China)). The temperature is measured by a platinum-rhodium – platinum thermocouple (TCP-0679 886) and a measuring and regulating instrument METACON RS-485.
The furnace is preliminary heated within 3,5-4 hours. Then 50-60% of a charge is loaded into the furnace bath. After melting of the first portion of the charge the remained part is loaded and fused within 45-60 minutes.
The carbide formation is controlled by means of sampling of the melt from the bath by the rod made from reinforcing steel. Primarily the notch is taped by a steel rod, and then it is burnt by the electric arc created by the special device connected to the transformer. The calcium carbide and ferroalloy unloading is realized simultaneously in a casting mold. After the unloading of the melt the charge is again loaded into the bath according to the procedure described above. After cooling the melt is sorted on calcium carbide and a ferroalloy. Quality of the calcium carbide produced is determined by its capacity. The capacity may be calculated under the formula [18]:
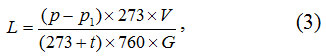
Where p иp1 –atmospheric pressure and water vapor tension during the experiment, mm Hg;
Va – volume of the acetylene liberated, ml;
G – calcium carbide mass, g;
T – temperature, °С;
L – calcium carbide capacity, l/kg.
Content of CaC2 in the commercial calcium carbide is calculated according to the formula:
![]()
where 372 – quantity of liters of the acetylene formed from 100% calcium carbide at 20°С and 760 mm Hg.
Extraction degree of Zn into the sublimates is calculated under the formula:
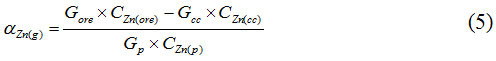
Where Gore, Galloy, Gcc – weights of the ore, the alloy and the calcium carbide, g;
СZn(ore), CZn(alloy), CZn(cc) – Zn content in the ore, the alloy and the calcium carbide.
Si content in the alloy (CSi) may be determined using the ferroalloy density ([rho]) under the formulas: at the density from 3,52 to 6,09 g/cm3:
Csi = 0,859 x p2-21,232 x p + 130,878 (6)
at the density from 6,09 to 7,859 g/cm3:
Csi = 9, 515 x p3-208,001 x p2 + 1524,918 (7)
Check of the Si content in the alloy is realized by means of a scanning electron microscope JOIL (Japan). For preliminary determination of the elements’ behavior we have carried out thermodynamic modelling of the process with use of aHSC-5.1 program [16].
The experiments have been performed with two kinds of the charge (table 2).
Table 2: The charge composition
|
Charge # |
Raw material |
Content |
||
|
% |
kg |
|||
|
1 |
Ore |
67,9 |
25,0 |
|
| Coke |
27,1 |
10,0 |
||
| Quartzite |
5 |
1,8 |
||
|
2 |
Ore |
59,9 |
25,0 |
|
| Coke |
29,0 |
13,0 |
||
| Quartzite |
4,0 |
1,8 |
||
| Lime |
11,1 |
5,0 |
||
For obtaining a ferroalloy with Si content > 20% both the compositions included quartzite. For production of a high-quality calcium carbide the second charge contained lime.
Results And Discussion
Figure 2 represents the temperature effect on the quantitative distribution (in terms of 100 kg of the ore) of Si, Ca, Zn and Pb in the substances forming the ferroalloy, calcium carbide and sublimates. (This information has been obtained by means of the HSC-5.1 program). As follows from the figure, СаС2 in the system of the ore – C is formed at Т≥1800°С (with a maximum at 2000°С), FeSi – at 1500°С, Si – at 1500°С, Fe3Si – at 1400°С and Fe5Si3 – at 1700°С; Zn completely passes in a gas phase at Т≥1800°С, and Pb – at 2300°С.
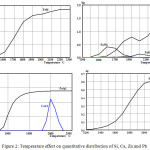 |
Figure 2: Temperature effect on quantitative distribution of Si, Ca, Zn and Pb |
The operating mode of electrosmelting the first mixture is represented in figure 3. As appears from the figure,the current intensity during theelectrosmelting fluctuated from 450 to 770А, pressure from 20 to 37V. The temperature under the furnace roof after the charge loading was 760-813°С. The electrosmelting the second mixture has been realized in the similar conditions.
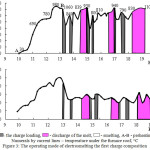 |
Figure 3: The operating mode of electrosmelting the first charge composition |
 |
Figure 4: The products obtained at the electrosmelting the Achisay ore: |
1 and 2 – the ferroalloys obtained at the electrosmelting the first and second compositions; 3 and 4 – the calcium carbide obtained at the electrosmelting the first and second compositions
The products obtained at the electrosmelting the Achisay ore (the ferroalloy and calcium carbide)are represented in figure 4;Si+Al content in the alloy (calculated through the alloy density) and the calcium carbide capacity (L) are shown in table 3.
Table 3: The content of Si and Al in the samples of alloys and the capacity of calcium carbide
|
1 charge composition |
Si+Al, % |
36,3 |
13,4 |
29,6 |
22,1 |
24,5 |
29,6 |
25,6 |
|
L, l/kg |
161 |
254 |
260 |
296 |
311 |
130 |
180 |
|
|
2 charge composition |
Si+Al, % |
21,9 |
29,9 |
22,1 |
26,5 |
36,4 |
25,3 |
27,6 |
|
L, l/kg |
286 |
244 |
289 |
208 |
266 |
300 |
332 |
As follows from the table 3, the ferroalloy in both cases contains 21,9 and 36,9% of Si+Al. The calcium carbide capacity in the first case is 161-311 l/kg, and in the second case, predictably, it is higher – 208-332 l/kg. Zinc content in the sublimates has been determined by a trilonometric method amounted to 41,93%
The scanning electron microscopy of the some produced alloys and sublimates is represented in figures 5 and 6. The analysis has shown that the alloy contains 22,09-24,43% of Si+Al (table 4), and the sublimates 44,3% of Zn, 1,76% Pb (table 5).
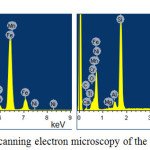 |
Figure 5: Scanning electron microscopy of the ferroalloys |
Table 4: Elemental composition of the alloys
|
С, % |
Mg |
Al |
Si |
Ca |
Ti |
Cr |
Mn |
Fe |
Ni |
|
Alloy 1 |
0,11 |
1,57 |
20,52 |
0,45 |
0,45 |
14,75 |
0,25 |
61,6 |
0,3 |
|
Alloy 2 |
0,26 |
1,78 |
22,75 |
0,59 |
0,45 |
4,77 |
0,22 |
69 |
0,17 |
Note: *C – content of elements
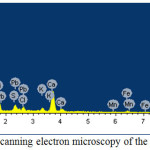 |
Figure 6: Scanning electron microscopy of the sublimates |
Table 5: Elemental composition of the sublimates
|
С, % |
O |
Mg |
Al |
Si |
S |
Cl |
K |
Ca |
Mn |
Fe |
Zn |
Pb |
|
Sublimates |
29,33 |
14,78 |
0,61 |
1,72 |
0,68 |
0,69 |
0,63 |
4,66 |
0,16 |
0,63 |
44,34 |
1,76 |
Note: *C – content of elements
During the experiments the silicon extraction degree in the alloy has made 84-88%, calcium in the calcium carbide 78-82%, zinc in the sublimates 99,4-99,8%, lead 99,1-99,6%, iron in the alloy 94-96%.
It is necessary to notice, that the suggested technology is characterized by higher level of complex processing the ore ([gamma]). So, at the Waelz processing about 87% of Zn and 91,5% of Pb are extracted in sublimates, and calcium, silicon and iron mainly pass in a waste clinker and only 0,8-1,2% (on the average 1%) of these elements pass in sublimates. In this case [gamma] makes:
γ = (87 +91,5 + 3 x 1)/ 5 = 36,3% (8)
In our case a level of complex processing the ore in terms of 5 elements is much higher and makes:
γ= (99,6 +99,3 + 80 + 89 1 + 95)/5 = 91,98% (8)
I.e. this factor increases in 91,88/36,3=2,53 times.
Besides the combination of three processes in one technological unit (an electric furnace) allows to reduce thermal processes in 2 times. At the expense of it the electric power consumption per a unit of the processed ore decreases approximately on 4-6%. If to process 100 000 t of the ore per a year it is possible to decrease the energy costs will compose ≈ 1,5million USD
Conclusion
The results obtained at the electrothermal processing of the mixture of Achisay rich and poor ores allow to draw following conclusions:
In equilibrium conditions zinc completely passes in the sublimates at Т≥1800°С, and lead at 2300°С. Maximum calcium transition in calcium carbide is observed at 2000°С. Silicon starts to pass in a marked degree in the alloy as Si+Al, FeSi, Fe3Si and Fe5Si3 at 1400-1700°С.
At the ore electrofusion there is formation of the ferroalloy containing 22,09-24,43% of Si+Al, the calcium carbide with capacity of 161-332 l/kg and the zinc sublimates including 44,34% of Zn and 1,76% of Pb.
The suggested technology in comparison with the Waelz process allows to increase a level of complex processing the ore in 2,52 times, to reduce thermal losses and power consumption on 1,5 million USD (at annual processing 100 000 tonn of the ore).
Acknowledgment
This work was supported by the Ministry of Education and Science of the Republic of Kazakhstan for grant financing on the theme “Non-waste technology of processing carbonate zinc-containing ores with production of ferroalloys, calcium carbide and zinc-containing sublimates”.
References
- AbdeevM.A.;KolesnikovA.V.;UshakovN.I.Wellawayazinc-lead-containingmaterials. Moscow: Metallurgy, 1985, pp: 120.
- ShevkoM.V.;KarataevaG.E. Metallurgy of zinc and cadmium. Shymkent: SKSU, 2015, pp: 350.
- PalenovaE.E.;BelogubE.V.;Kotlyarov V.A.Mineralogy of oxidized ores of the Deposit Shaimerden. In the XI Congress of the pod. Modern Mineralogy: from theory to practice. St. Petersburg, 2010, pp: 231-233.
- Methodical recommendations on application of Classification of reserves and prognostic resources of solid minerals “Lead and zinc ore”, Moscow, 2007, pp: 40.
- Romanteev Y.P.;Fedorov A.N.; Bystrov S.V.Metallurgy of zinc and cadmium. Moscow: MISiS, 2006, pp: 193.
- Antropova I.G.; Gulyashinov A.N.; Lamuev V.A.; Paleev P.L.Method of processing refractory oxidized lead ore, Patent Russian Federation #2364639, 2009.
- Gulyashinov A.N.; Antropova I.; Kalinin Y.O.; Khanturgaeva G.I. Method for processing of oxidized zinc ore, Patent Russian Federation #2208059, 2003
- Antropova I.G. Physico-chemical and technological bases sulfidizing firing of oxidized lead-zinc ore in the atmosphere of superheated steam: author. dis. kand. tech. Sciences. Krasnoyarsk,2005, pp: 40-42.
- Gurov V.A.; Ivanov V.S.; Shulgin A.S. Feature extraction of manganese from oxide and carbonate-silicate ores they discovered the parnokskoye Deposit in relation to underground leaching sulfuric acid solutions.GORN,2003; 9: 134-139.
- New methods for processing zinc-containing raw materials abroad: a review.inform. The production of heavy non-ferrous metals. Moscow: CRIEICM, 1974, pp: 44.
- Kazanbaev L.A.; Kozlov P.A.; Kubasov V.L. Hydrometallurgy of zinc. The process of leaching. Moscow: Ore and metals, 2007, pp: 120.
- Kim L.D.; Tulyaganov S.R.; Mamatkulov H.; Filipcev A. A.; Mavlonkulov R. K.; Choi Y.; Mamatkulov H. P.Method for processing poor oxidized zinc ores and concentrates with the recovery of zinc, manganese, iron, lead, silver, calcium and silicon dioxide, Patent Russian Federation #2441930, 2010
- Shevko V. M.; Badikova A.D.; Serzhanov G. M.;Karataeva G. E.; Tuleev M. A. Complex electro-thermal processing of Kazakhstan’s oxidic zinc-containing ores of Achisay deposit. International Journal of Applied Engineering Research,2016; 11(22): 10838-10841.
- Shevko V. M.; Tuleev M. A.; Karataeva G. E.;Aytkulov D. K.Determination of the optimal temperature and time of the electric smelting of ore Achisay obtaining Ferroalloy, calcium carbide and Stripping of zinc. Works of international scientific-practical conference “Auezov readings – 14: Innovative potential of science and education of Kazakhstan into the new global reality”.Shymkent: SKSU named after M. Auezov, 2017, pp: 272-275
- Shevko V.M.; Bishimbayev V.K.; Serzhanov G.M.; Kolesnikov A.S.; Tuleev M.A.Method for processing zinc-containing ores oxide, Innovative Pat. #26393, The Republic Of Kazakhstan, 2012
- Roine A.Outokumpu HSS Chemistry for Windows. Chemical Reaction and Eguilibriumloftware with Extensive Thermochemical Database. Pori: Outokumpu Research OY, 2002
- Shevko V.M. Wasteless technology of processing zinc-containing carbonate ores to obtain ferro-alloys, calcium carbide and zinc-containing sublimates: research Report (interim), 2015-2017. Shymkent, SKSU, state registration #0115РК011506; inv 0216РК00437, 2016, pp: 167.
- Kozlov K.B.; Lavrov B.A.Production of calcium carbide in an arc furnace and its analysis. St. Petersburg: SPSIT (TU), 2011, pp: 24.

This work is licensed under a Creative Commons Attribution 4.0 International License.









