Manganese(III) Acetate Mediated Synthesis of 3-Arylsulfenylindoles and Evaluation of Their Antibacterial Activity
Ravi Kumar G.M.V.N.A.R and Thangamani Arumugam
Department of Chemistry, Karpagam University, Karpagam Academy of Higher Education, Coimbatore-641021, Tamil Nadu, India.
Corresponding Author E-mail: thangabell2003@gmail.com
DOI : http://dx.doi.org/10.13005/ojc/340150
The present work reports a simple and efficient method for the sulfenylation of a wide variety of indoles with aryl, benzothiazolethiols using manganese(III) acetate promoted free radical reaction. This method is selective at the C3 position of indoles and offers several advantages such as broad substrate scope, functional group tolerance (bromo, carboxylic acid, methoxy, difluoromethoxy, ester groups) and gives the required products in good to excellent yields. The experimental simplicity makes it a useful and attractive approach for the synthesis of 3-aryl sulfenyl indoles. The compounds 1-12 are evaluated for the anti-bacterial agents. Most of them exhibited promising activities.
KEYWORDS:Sulfenylation of Indoles; Regioselectivity; Manganese(III) Acetate; C-S Bond Formation; Antibacterial Activity
Download this article as:| Copy the following to cite this article: Kumar R. G. M. V. N. A. R, Arumugam T. Manganese(III) Acetate Mediated Synthesis of 3-Arylsulfenylindoles and Evaluation of Their Antibacterial Activity. Orient J Chem 2018;34(1). |
| Copy the following to cite this URL: Kumar R. G. M. V. N. A. R, Arumugam T. Manganese(III) Acetate Mediated Synthesis of 3-Arylsulfenylindoles and Evaluation of Their Antibacterial Activity. Orient J Chem 2018;34(1). Available from: http://www.orientjchem.org/?p=42645 |
Among the most important heterocyclic units indole nucleus has received major attention as a key structural building block due to their medicinal and pharmaceutical applications.1 Further 3-thioindole motifs are very common in various drugs for the treatment of heart disease,2 allergies,3 these are also utilized in the field of organic nonlinear optical materials.4 The following compounds such as MK-886 (III) is having antitumor activity,5 L-737,126 (VII) is identified as a potent anti-HIV agent,6 the 3-(arylsulfenyl)indole is able to inhibit human breast cancer cell growth,7 vasoconstrictor tinazoline (I) is 4,5-dihydro-2- (3-indolyl) mercaptoimidazole known as a nasal decongestant8 and acting as an alpha-adrenergic blocking drug (Figure 1).
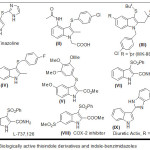 |
Figure 1: Biologically active thioindole derivatives and indole-benzimidazoles Click here to View figure |
It is planned to synthesize analogues of tinazoline by replacing the imidazole moiety with benzimidazole because compounds containing benzimidazole have numerous biological activities, for example antitumor9 and antiparasitic10. Although compounds containing both indole-benzimidazoles are known to show diuretic acitivity11 but their complete study is unexplored and hence this work targets to synthesize Compounds containing both indole-benzimidazoles (or benzothiazole ) through C-S bond.
Recently, various methods are reported for the 3-arylthiolation of indoles using the various sulfenylating agents such as buntesalts,12 disulfides,13sulfenyl halides,14 and N-thioarylphthalimides.15 Reagents such as Vanadium oxyacetylacetonate,16 AlCl317,Selectfluor18, NCS19, oxone20, FeF321, (bis(trifluoroacetoxy)iodo benzene)22, Cu23 and Iodine24, and CeCl330 have been reported under different conditions. Particularly substrates substituted benzimidazolethiol and benzothiazolethiol were unexplored in the 3-arylthiolation. Therefore, the development of a simple, convenient and general methodology for the sulfenylation of indoles utilizing a stable and readily available reagent would extend the scope of this reaction and further applications of 3-arylsulfenyl indole products.
Although Mn(OAc)3 is a good single-electron oxidant, selective, mild oxidant and commercially available. Use of Mn(III)acetate in C-C bond construction between carbon centered radical with olefins25 and arenes26 was well investigated. But its applications are limited mostly in the field of sulfur-centered free radicals generating from thiols.27 The main advantage in the selection of this reagent is its moderate reactivity thereby is envisioned higher selectivity in the thioarylation.
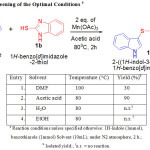 |
Table 1: Screening of the Optimal Conditionsa
|
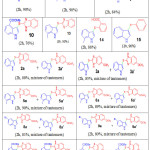 |
Table 2: reaction of various indoles with different thiols using Mn (OAc)3. Click here to View table |
Material and Methods
Initially we conducted reaction between 1H-indole and 1H-benzoimidazole thiol using Mn(OAc)3and tried to optimize the reaction conditions (Table 1). In the beginning, this reaction is conducted under solvent free conditions followed by preferred solvents such as water and Ethanol. But good results were not observed in the reaction. Then reaction is examined with toluene, acetonitrile, THF, DMF and acetic acid28. Among the different solvents surveyed, better yield was observed with Acetic acid and moderate yield in DMF. Acetic acid is the solvent of choice, because it has a good solubility for Mn(OAc)3 and also giving higher yields in addition to that it degrades rapidly to harmless substances in the environment.
Results and Discussion
The optimized condition for regeoselectivesulfenylation reaction of indoles consists of using Indole (1.0equiv.), thiol (1.0 equiv.), reagent (2 equiv.) in acetic acid at 800C for 2hours (Table-1, entry 2).With this optimized reaction condition, the scope of the reaction is extended by taking into account various thiols with indole derivatives. As shown in the Table 2, unsubstituted indole with thiol yielded with the required products (85- 90%, entries 1-3),indoles with electron-donating methyl group (88-92%; Table entries 4,5,6,13 & 14) resulted higher yields in comparison to those having electron-withdrawing groups(72-76%; Table 2, entries 10-12). The reaction is tolerant to bromo substituent on the aromatic ring of indole, and the corresponding target products were obtained in good yields (80-84%; Table 2, entries 7-9). Compounds 2a, 3a,5a, 6a,8a, 9a, 11a and 12a, are existing as a mixture of tautomers.29
Plausible Mechanism
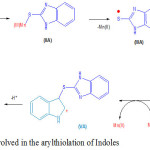 |
Scheme 2: Mechanism in volved in the arylthiolation of ln doles Click here to View scheme |
A plausible mechanism27c is proposed involving ligand-exchange pathway for the reaction of indole with 1H-benzo[d]imidazole-2-thiol (1b) shown in the Scheme-2. The ligand exchange between Mn(OAc)3 and 1H-benzo[d]imidazole-2-thiol produces the intermediate (IIA), whichgets oxidized to the sulphur centered free radical (IIIA), and then attacks the electron-rich site of indole i.e. the 3ʹ-position to yield radical (IVA). Subsequently, Mn(OAc)3 oxidizes radical (IVA) to cation (VA) , and finally loses a proton affording 3-arylthiol product (1).
Experimental Section
Chemistry
Melting points were determined in open capillaries and are uncorrected. IR spectra (KBr) were recorded on a Jasco, FT / IR–4100 spectrophotometer. Mass spectras were recorded on Shimadzu, LC-2010EV. 1H NMR and 13C NMR spectra were recorded on JEOL 400MHz and Brukar Avance 300 MHz spectrometer. TMS was used as internal reference. All the analysis data obtained at Sapala Organics Private Limited, Hyderabad.
Materials and Methods
Manganese(III) acetate mono hydrate used for these reactions was purchased from Aldrich. All the other solvents and raw materials were purchased from common suppliers. Reactions were monitored by thin-layer chromatography (TLC) on silica gel plates (60A˚; Aldrich), visualized under 254 nm ultraviolet Light. Column chromatography separations were performed using silica gel (70–230 mesh) obtained from the Aldrich Company. The solvents used for elution varied depending on the compound and included either one or a combination of hexane and ethylacetate. All reactions were conducted under a nitrogen atmosphere unless otherwise noted.
Typical experimental procedure for the synthesis of Compounds 1-14.
Manganese triacetate (2 mmol) is added portion wise to a pre dissolved solution of Indole (1mmol) and benzimidazolethiol (1mmol) in acetic acid (10mL) at room temperature under Nitrogen atmosphere. The reaction mixture is stirred at 800C for 2hr. After completion of reaction, as monitored by T.L.C analysis, the reaction mixture was quenched by the addition of water 20ml. The organic compounds are extracted with ethyl acetate (3x20ml). The combined layers are dried over anh.Na2SO4. The required product is purified by column Chromatography, eluted with 8% methanol in DCM to get the product. The product is confirmed by 1H, 13C, NMR, IR and mass.
2-(1H-indol-3-ylthio)-1H-benzo[d]imidazole (Compound 1)
90% Yield; m.p. 2210C; Rf= 0.750 (Hexane:Ethyl acetate, 60:40, v/v);1H NMR (400 MHz, DMSO-d6, 25°C) δ ppm 8.32 – 8.43 (3 H, m), 8.52 (1 H, t, J=7.44 Hz), 8.66 (2 H, br s), 8.79 (1 H, d, J=8.1 Hz), 8.83 – 8.86 (1 H, m), 9.21 (1 H, d, J=3.1 Hz), 13.13 (2 H, br s); 13C NMR (101 MHz, DMSO-d6) δ ppm 95.6, 112.3, 118.1, 120.2, 121.2, 121.2, 122.1, 128.8, 133.0, 136.6, 150.9; IR: 3381cm-1 (N-H); ESI-Mass: m/z 266[M. ++1 peak]; Mol. Formula:C15H11N3S;
2-(1H-indol-3-ylthio)-6-(difluoromethoxy)-1H-benzo[d]imidazole & 2-(1H-indol-3-ylthio)-5-(difluoromethoxy)-1H-benzo[d]imidazole (Compound 2 as tautomeric mixture of 2a and 2a’)
88% Yield; m.p. 209-2110C 0C; Rf= 0.77 (Hexane: Ethyl acetate, 60:40, v/v); 1H NMR (400MHz, DMSO-d6,25°C) δ 6.90-6.93 (m, 1H), 7.05-7.36(m , 4H), 7.45 -7.52 (d, J = 7.8 Hz, 4H), 7.91 (1 H, d, J = 2.3 Hz), 11.82 (br s, 1H); 13C NMR (100MHz, DMSO-d6) δ 95.2, 112.3, 113.6, 116.8, 118.0, 120.3, 122.2, 128.7, 133.2, 136.6, 145.7, 202.3; IR: 3411cm-1 (N-H); ESI-Mass: m/z 332 [M. ++1 peak]. Mol. Formula: C16H11F2N3OS
2-(1H-indol-3-ylthio)-6-methoxy-1H-benzo[d]imidazole & 2-(1H-indol-3-ylthio)-5-methoxy-1H-benzo[d]imidazole (compound 3 as tautomeric mixture of 3a and 3a’)
85% Yield; m.p. 219-2210C 0C; Rf= 0.77 (Hexane: Ethyl acetate, 60:40, v/v);1H NMR (400 MHz, DMSO-d6, 25°C) δ ppm 3.71 (s, 3H), 6.68 (dd, J= 8.6, 2.3 Hz, 1H), 6.86 (br s, 1H), 7.07 – 7.11(m, 1H), 7.15 – 7.29 (m, 2H), 7.47 (br d, J = 7.82 Hz, 1H), 7.51 (d, J = 7.83 Hz, 1H), 7.87 (d, J = 2.35 Hz, 1H), 11.78 (br s, 2H); 13C NMR(100 MHz, DMSO-d6) δ ppm 55.3, 96.1, 110.3, 112.2, 118.1, 120.1, 122.1, 128.8, 132.8, 136.6, 155.1; IR: 3363cm-1 (N-H); ESI-Mass: m/z 296[M. ++1 peak]; Mol. Formula: C16H13N3OS
2-(7-methyl-1H-indol-3-ylthio)-1H-benzo[d]imidazole (compound 4)
83% yield; m.p. 1830C; Rf= 0.49 (Hexane: Ethyl acetate, 70:30, v/v); 1H NMR(300 MHz, DMSO-d6, 27°C) δ ppm 2.55 (3 H, s), 6.98 – 7.06 (4 H, m), 7.22 – 7.29 (2 H, m), 7.40 – 7.43 (1 H, m), 7.90 (1 H, d, J=2.7 Hz), 11.77 (1H, s), – 11.83 (1 H, s); 1H NMR (300 MHz, CDCl3, 27°C) δ ppm 2.56 (3 H, s), 7.03 – 7.19 (6 H, m), 7.47 – 7.52 (2 H, m), 8.38 (1 H, bs); 13C NMR (500 MHz, DMSO-d6) δ ppm 97.5, 115.5, 118.0, 118.0, 121.5, 121.9, 123.0, 123.9, 126.1, 127.7, 133.5, 154.0, 171.4; ESI-Mass: m/z 280 [M.++1 peak]; Mol. Formula: C16H13N3S
2-(7-methyl-1H-indol-3-ylthio)-6-methoxy-1H-benzo[d]imidazole & 2-(7-methyl-1H-indol-3-ylthio)-5-methoxy-1H-benzo[d]imidazole (compound 5 as tautomeric mixture of 5a and 5a’)
90% yield; m.p. 1830C; Rf= 0.6 (Hexane: Ethyl acetate, 70:30, v/v);1H NMR (400 MHz, DMSO-d6, 25°C) δ ppm 2.45 (6 H, s), 3.30(6 H, s), 3.66(6 H, s), 3.77(6 H, s), 6.45(1 H, d, J=2 Hz), 6.57(1 H, d, J=2 Hz), 6.57(1 H, d, J=2 Hz), 6.74-6.79 (2H, m),6.83-6.87 (2H, m), 6.97-7.01 (5H, m), 7.16 (1H, d, J=8.8Hz), 7.42-7.47 (2H, m); 13C NMR (101 MHz, DMSO-d6) δ ppm 16.7, 55.6, 55.6, 94.7, 94.7, 99.5, 99.7, 109.9, 110.2, 110.3, 110.9, 118.0, 118.1, 119.6, 120.8, 120.9, 122.5, 122.5, 124.9, 126.4, 126.4, 128.2, 128.4, 128.5, 131.6, 134.0, 134.1, 135.1, 156.1, 156.6, 170.1; IR: 3410cm-1 (N-H); ESI-Mass: m/z 310 [M. ++1 peak]; Mol. Formula: C17H15N3OS
2-(7-methyl-1H-indol-3-ylthio)-6-(difluoromethoxy)-1Hbenzo[d]imidazole & 2-(7-methyl-1H-indol-3-ylthio)-5-(difluoromethoxy)-1H-benzo[d]imidazole (compound 6 as tautomeric mixture of 6a and 6a’)
89% Yield, m.p. 1100C, Rf= 0.77 (Hexane: Ethyl acetate, 60:40, v/v); 1H NMR (400 MHz, DMSO-d6, 25°C) δ ppm 2.53 – 2.54 (4 H, m), 6.89 – 6.93 (2 H, m), 6.98 – 7.04 (3 H, m), 7.10 (2 H, s), 7.90 (1 H, d, J=3.1 Hz), 11.67 – 12.07 (2 H, m) 13C NMR (101 MHz, DMSO-d6) δ ppm 16.5, 58.2, 95.5, 113.6, 114.3, 115.6, 116.8, 120.5, 121.6, 122.7, 128.5, 132.9, 136.1, 145.7, 152.7; IR: 3402cm-1 (N-H); ESI Mass: m/z 346 [M.++1 peak]; Mol. Formula: C17H13F2N3OS
2-(5-bromo-1H-indol-3-ylthio)-1H-benzo[d]imidazole (compound 7)
84% yield, m.p. 268-2700C, Rf Value 0.86 (10% methanol in DCM); 1H NMR (400 MHz, DMSO-d6, 25°C) δ ppm 7.06 -7.08 (2 H, m), 7.30 – 7.35 (3 H, m), 7.47 – 7.49 (1 H, d , J=8.4), 7.57 (1H, d, J=1.6), 7.93 (1H, d, J=2.8), 11.99 (2H, bs); 13C NMR (101 MHz, DMSO-d6) δ ppm 95.2, 113.0, 113.7, 116.8, 114.5, 120.2, 124.8, 130.7, 134.8, 135.4, 145.8; IR: 3392cm-1 (N-H); ESI-Mass: m/z 344 [M. ++1 peak]; Mol. Formula: C15H10BrN3S
2-(5-bromo-1H-indol-3-ylthio)-5-(difluoromethoxy)-1H-benzo[d]imidazole & 2-(5-bromo-1H-indol-3-ylthio)-6-(difluoromethoxy)-1H-benzo[d]imidazole (compound 8 as tautomeric mixture of 8a and 8a’)
81% Yield, m.p. 2400C, Rf =0.51 (in 10% methanol in DCM); 1H NMR (400 MHz, DMSO-d6) δ ppm 6.97(1 H, dt, J=5.8, 2.5 Hz), 7.16 (1 H, s), 7.22(1 H, br s), 7.30 – 7.48(3 H, m), 7.55(1 H, d, J=8.6 Hz), 7.63(1 H, d, J=1.5Hz), 8.02(1 H, d, J=2.3Hz), 11.96 – 12.21(2 H, m); 1H NMR (400 MHz, CD3OD) δ ppm 6.69(1H, t, 74.4 Hz), 6.91 (1H, d, J=8.8Hz), 6.93 (5H , d , J= 8.8)13C NMR (101 MHz, DMSO-d6) δ ppm 95.6, 112.9, 114.4, 120.2, 121.3, 124.7, 130.8, 134.6, 135.3, 150.38; IR: 3398cm-1 (N-H); ESI-Mass: m/z 411 [M.++1 peak]; Mol. Formula: C16H10BrF2N3OS
2-(5-bromo-1H-indol-3-ylthio)-5-methoxy-1H-benzo[d]imidazole & 2-(5-bromo-1H-indol-3-ylthio)-6-methoxy-1H-benzo[d]imidazole (compound 9 as tautomeric mixture of 9a and 9a’)
80% Yield, m.p. 1900C, Rf Value 0.47 (in 10% methanol in DCM), 1H NMR (400 MHz, DMSO-d6, 25°C) δ ppm 3.70(3H, s), 6.69(1H, dd, J= 8.8Hz, J=2.4Hz), 6.86 (1H, bs), 7.23(1H, d, J=7.2 Hz), 7.3(1H, dd, J=8.4, J=3.2 Hz), 7.47(1H, d, J=7.2 Hz), 7.57 (1H, s), 7.91 (1H, d, J=2.8Hz), 11.82 (1H, s), 11.95(1H, s); 13C NMR (101 MHz, DMSO-d6) δ ppm 55.3, 95.2, 96.0, 106.0, 110.4, 112.9, 114.4, 120.2, 124.7, 130.7, 134.4, 135.3, 155.1; IR: 3389cm-1 (N-H); ESI mass: m/z 373[M.++1 peak]; Mol. Formula: C16H12BrN3OS
methyl 3-(1H-benzo[d]imidazol-2-ylthio)-1H-indole-4-carboxylate (compound 10)
76%Yield, m.p. 2400C, Rf Value 0.54(in 10% methanol in DCM); 1H NMR (400 MHz, DMSO-d6) δ ppm 1.07 – 1.27 (m, 1 H), 3.67-3.95 (m, 4 H), 7.01 – 7.13 (m, 2 H), 7.23 – 7.34 (m, 1 H), 7.40 – 7.50 (m, 1 H), 7.63 (d, J=8.6Hz, 1H), 7.84 (dd, J=8.6, 1.5 Hz, 1H), 8.04 – 8.16 (m, 2H), 11.97 (s, 1H), 12.19 (br s, 1H); 13C NMR (101 MHz, DMSO-d6) δ ppm 51.7, 97.4, 110.5, 112.5, 117.4, 120.4, 121.1, 121.5, 121.7, 123.1, 128.5, 135.2, 139.3, 143.9, 150.4, 166.8; IR: 1688cm-1 (ester, C=O stretching), 1618 (ester, C=O asymm. Str.);ESI Mass: m/z 324 [M. ++1 peak]; Mol. Formula: C17H13N3O2S
methyl 3-(5-methoxy-1H-benzo[d]imidazol-2-ylthio)-1H-indole-4-carboxylate & methyl 3-(6-methoxy-1H-benzo[d]imidazol-2-ylthio)-1H-indole-4-carboxylate (compound 11 as tautomeric mixture of 11a and 11a’)
76% Yield, m.p. 2200C, Rf Value 0.60(in 10% methanol in DCM); 1H NMR (400 MHz, DMSO-d6, 25°C) δ ppm 1.15 – 1.35 (1 H, m), 3.29 (1 H, br s), 3.36 (3 H, br s), 3.70 – 3.90 (7 H, m), 6.71 (1 H, d, J=8.8Hz), 6.87 (1 H, br s), 7.24 (1 H, br d, J=8.6 Hz), 7.62 (1 H, d, J=8.6Hz), 7.84 (1 H, dd, J=8.6, 1.5Hz), 8.04 (1 H, d, J=2.3Hz), 8.16 (1H, s), 12.17 (1H, br s); 13C NMR (101 MHz, DMSO-d6) δ ppm 51.7, 55.3, 97.8, 110.4, 112.4, 120.4, 121.7, 123.0, 128.5, 135.0, 139.3, 155.2, 166.8; IR: 1685cm-1 (ester, C=O symm. str.), 1619 (ester, C=O asymm. Str.) ESI Mass: m/z 354 [M. ++1 peak]; Mol. Formula: C18H15N3O3S
methyl 3-(5-(difluoromethoxy)-1H-benzo[d]imidazol-2-ylthio)-1H-indole-4-carboxylate and methyl 3-(6-(difluoromethoxy)-1H-benzo[d]imidazol-2-ylthio)-1H-indole-4-carboxylate (compound 12 as tautomeric mixture of 12a and 12a’)
72% yield, m.p. 205-2070C, Rf Value 0.45(in 10% methanol in DCM); 1H NMR (400 MHz, DMSO-d6, 25°C) δ ppm 3.74 (1 H, s), 3.78 – 3.86 (3 H, m), 6.93 (1H, br d, J=6.2Hz), 7.11 (1H, s), 7.17 (1H, br s), 7.24 – 7.44 (1H, m), 7.64 (1H, br d, J=8.6Hz), 7.85 (1H, br d, J=7.8Hz), 8.04 – 8.11 (1H, m), 8.11 – 8.17 (1 H, m), 11.99 – 12.27 (2 H, m); 13C NMR (101 MHz, DMSO-d6) δ ppm 51.5, 51.7, 97.0, 112.5, 113.7, 120.3, 121.8, 123.1, 124.8, 128.5, 135.3, 139.3, 145.8, 166.8; IR: 3312cm-1 (N-H), 1698 (ester, C=O symm. Str.), 1619 (ester, C=O asymm. Str.); ESI Mass: m/z 390 [M. ++1 peak]; Mol. Formula: C18H13F2N3O3S
2-(7-methyl-1H-indol-3-ylthio)benzo[d]thiazole (Compound 13)
92% Yield, m.p. 2100C, Rf Value 0.80 (10% methanol in DCM) 1H NMR (300 MHz, DMSO-d6, 27°C) δ ppm 2.54 (3 H, s), 7.03 (2 H, m), 7.22-7.28 (1H, m), 7.35-7.43 (2H, m), 7.8 (2H, d, J= 8.7Hz), 12.04 (1H, bs); 1H NMR (300 MHz, CD3OD-d6, 27°C) δ ppm 2.58 (3 H, s), 7.06 – 7.11 (2 H, m), 7.23-7.28 (1H, t, J=7.2Hz), 7.39-7.44 (2H, m), 7.65-7.68 (1H,d, J=8.1Hz), 7.75 -7.78 (2H, m); C13NMR (500 MHz, DMSO-d6, 27°C) 16.5, 97.5, 115.5, 121.02, 121.0, 121.5, 121.9, 123.0, 123.9, 126.1, 127.7, 133.5, 154.0, 173.4; ; IR: 3418cm-1 (N-H); ESI Mass: m/z 297 [M. ++1 peak]; Mol. Formula: C16H12N2S2
2-(7-methyl-1H-indol-3-ylthio)benzoic acid (compound 14)
88% Yield, m.p.1430C, Rf Value 0.61(10% methanol in DCM); H1 NMR (300 MHz, CD3OD, 27°C) δ ppm 2.58 (3H, s), 7.73 – 7.76 (1H, dd, J=0.9Hz, J=6Hz), 6.92-6.98 (2H, m), 7.04 (1H,t, J=6.3), 7.10 (1H, t, J=5.7), 7.98 (1H, d, J=5.7), 7.48 (1H, m), 7.96 (1H, dd, J=6, J=1.2), 11.09 (1H, bs) ESI Mass: m/z 284[M. ++1 peak]; Mol. Formula: C16H13NO2S
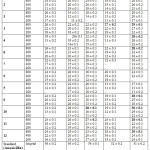 |
Table 3: Anti-bacterial activity ((Cup-plate Method) Click here to View table |
Anti-Bacterial Activity
The newly synthesized compounds 1 to 12 are screened for antibacterial activity against gram-positive bacterium (B. subtilis and S. aureus) and Gram-negative bacteria (P. aeruginosa, E. coli and S. typhi). Dimethyl formamide is used as solvent control. The bacterial culture was inoculated on nutrient agar (Merck) (20 mL) and poured into sterilized Petri dishes (99 mm). Media plates were inoculated with liquid cultures homogeneously by spread plate method. All the test compounds were dissolved in dimethyl formamide(DMF) to get different concentration of 100 µL and loaded into the wells of agar plates directly. Plates inoculated with the bacteria were incubated at 37oC for 24h. All determinations were done in triplicates. Amoxicillin (1mg/mL) were used as standard drugs for antibacterial.
The results for antibacterial activities depicted in Table 3. It reveals most of the compounds (1, 2, 4, 6, 8, 10, 11, and 12) showing good anti-bacterial activity against E.Coli. Incorporation of the ester group in the fourth position of the indole nucleus is favorable to anti-bacterial activity. Whereas the incorporation of Bromo or Methyl group on the indole nucleus is unfavorable to anti-bacterial activity.
Conclusion
A simple, convenient and inventive protocol for the sulfenylation of indoles using Mn(OAc)3 reagent was developed. This method offers several advantages such as broad substrate scope, high yields and regioselectivity. The experimental simplicity makes it a valuable and elegant strategy for the synthesis of 3-sulfenyl indoles.
Acknowledgments
We are thankful to the Management of Karpagam University to carry out my research work.
References
- For recent reviews on Indoles (a) Shinya S., Makoto, N.,Takanori, K. J Gastroenterol. 2016, 51, 853;
- Ming-Zhi, Z.; Qiong, C.; Guang-Fu, Y. Eur. J. Med. Chem.2015, 89, 421;
CrossRef - Douglass, F. T.; Pavan, K. T. Tetrahedron, 2011,67, 7195;
CrossRef - Humphrey, G. R.; Kuethe, J. T. Chem. Rev.2006, 106, 2875;
CrossRef - Van Zandt, M. C.; Jones, M. L.; Gunn, D. E.; Geraci, L. S.; Jones, J. H.; Sawicki, D. R.; Sredy, J.; Jacot, J. L.; Dicioccio, A. T.; Petrova, T.; Mischler, A.; Podjarny, A. D. J. Med. Chem.2005, 48, 314.
CrossRef - Funk, C. D.;Nat. Rev. Drug Discovery2005, 4, 664.
- Armer, R. E.; G. Wynne, M.;PCT Int. Appl. WO2008012511, 2008.
- Kawamonzen, Y.; Mori, Y.; Chem. Abstr.1993, 119, 237604.
- Cianchi ,F.; Cortesini, C.; Magnelli, L.; Fanti, E.; Papucci, L.; Schiavone, N.; Messerini, L.; Vannacci, A.; Capaccioli, S.; Perna, F.; Lulli, M.; Fabbroni ,V.; Perigli, G.; Bechi, P.; Masini; E. Mol. CancerTher. 2006, 5, 2716.
CrossRef - R Silvestri, M Artico, B Bruno, S Massa, E Novellino, G Greco, ME Marongiu, A Pani, A De Montis, P La Colla; Antiviral Chemistry & Chemotherapy 1998, 9(2), 139-148.
CrossRef - De Martino, G.; Edler, MC.; , La Regina, G.; Coluccia, A.; Barbera, MC.; Barrow, D.; Nicholson, RI.; Chiosis, G.; Brancale, A.; Hamel, E.; Artico, M.; Silvestri, R. J. Med. Chem.2006, 49, 947.
CrossRef - De Martino, G.; La Regina, G.; Coluccia, A.; Edler, MC.; Barbera, MC.; Brancale, A.; Wilcox, E.; Hamel, E.; Artico, M.; Silvestri, R. J. Med. Chem. 2004, 47, 6120.
CrossRef - Vaidya, A. B.; Paul, T. T.; Talwalkar, S. S.; Mankodi, N. A.; Sheth, U. K. J. Postgrad. Med.1983, 29, 133.
- Denny, W.A.; Rewcastle, G.W.; Baguley, B.C. J. Med. Chem. 1990, 33, 814.
CrossRef - Pabba, C.; Wang, H.J.; Mulligan, S.R.; Chen, Z.J..; Stark, T.M.; Gregg, B.T. Tetrahedron Lett. 2005, 46, 7553.
CrossRef - Radha, Y.; Manjula, K.M.; Reddy, B.M.; Rao, B.V. Ind. J. Chem. 2011, 50B, 1762.
- Hong, Q.; Tongxin, Z.; Kefeng, W.; Meiming, L. J. Org. Chem.2016, 81, 4262.
CrossRef - Azeredo, J. B.; Godoi, M.; Martins, G. M.; Silveira, C. C.; Braga, A. L. J. Org. Chem. 2014, 79, 4125.
CrossRef - Koval, I. V. Russ. Chem. Rev.1995, 64, 731.
CrossRef - Viglianisi, C.; Marcantoni, E.; Carapacchi, V.; Menichetti, S. Marsili, L. Eur. J. Org. Chem.2014, 2014, 6405.
- Maeda, Y.; Koyabu, M.; Nishimura, T.; Uemura. S. J. Org. Chem.2004, 69, 7688.
CrossRef - Ranken, P. F.; McKinnie, B. G. J. Org. Chem.1989, 54, 2985.
CrossRef - J. S. Yadav, B. V. Subba Reddy and Y. Jayasudhan Reddy; Tetrahedron letters2007, 48 (39), 7034-37.
CrossRef - Harriet Wall Hamilton, Alexei P. Krasutsky, Jessica Reed and Kevin Schlosser; US Patent Appl. 20040133014, 8th July 2004
- Guaili Wu, Jing Wu, Jialiang Wu, and Longmin Wu; Synthetic Communications 2008, 38, 1036–1043.
CrossRef - Xiao-Li Fang, Ri-Yuan Tang, Ping Zhong, Jin-Heng Li; SYNTHESIS2009, 24, 4183–4189,
- Campbell, J. A.; Broka, C. A.; Gong, L.; Walker, K. A. M.; Wang, J.-H. Tetrahedron Lett.2004, 45, 4073.
CrossRef - Jeffrey A. Campbell, Viola Bordunov, Chris A. Broka, Michelle F. Browner, James M. Kress, Tara Mirzadegan, Chakk Ramesha, Bong F. Sanpablo, Russell Stabler, Patricia Takahara, Armando Villasenor, Keith A. M. Walker, Jin-Hai Wang, Mary Welch and Paul Weller; Bioorg. Med.Chem.Lett.2004, 14, 4741–4745.
CrossRef - Zhimeng Wu, Yan-Chun Li, Wen-Zhang Ding, Tao Zhu, Shao-Zhong Liu, XuehongRen,and Liang-HuaZou; Asian J. Org. Chem.2016, 5, 625 – 628
CrossRef - Nian Zhou, Wayne Zeller, Michael Krohn, Herb Anderson, Jun Zhang, Emmanuel Onua, Alex S. Kiselyov, Jose Ramirez, Gułrun Halldorsdottir, Torkell Andresson, Mark E. Gurney, Jasbir Singh; Bioorganic & Medicinal Chemistry Letters 2009,19, 123–126.
CrossRef - Ayhan S. Demir and Mustafa Emrullahoglu; Current Organic Synthesis, 2007, 4, 223-237; (b) ReikaFujino, Hiroshi Nishino; Synthesis2005, 5, 0731–0740; (c) ManojMondala and Utpal Bora; RSC Adv., 2013,3, 18716-18754; (d) NobutakaKikue, Tetsuya Takahashi, and Hiroshi Nishino; Heterocycles2015, 90(1), 540 – 562.
- Che-Ping Chuang a andSheow-Fong Wang; Synthetic Communications,1994, 24(1l), 1493-1505;
- Dmitry A. Androsov, Tara L. S. Kishbaugh, GordonW. Gribble; Tetrahedron Letters2008, 49(47), 6621–6623;
- Verner A. Lofstrand, Bryan S. Matsuura, Laura Furst, Jagan M.R. Narayanam and Corey R.J. Stephenson; Tetrahedron2016, 72(26), 3775-3780.
- Nguyen, V.; Nishino, H.; Kajikawa, S.; Kurosawa, K.;Tetrahedron1998, 54, 11445;(b) M. V. Ramana Reddy, Muralidhar Reddy Mallireddigari; Bioorganic& Medicinal Chemistry2005, 13, 1715–1723; (c) Xiang, Q.P.; Mao,Y. L.; Jian,P. Z.; Wei, Z.;Tetrahedron Lett.2009, 50, 347.
- Kim, A.; Juan, C.; Peter, J. D.; Thomas, F.; Sandra, J.; Timothy, A. J.; Peter, K. H.; Craig, K.; Mark, A. N.; David, A. P.; Mark S.;Green Chem.2008, 10, 31.
CrossRef - For benzimidazole tautomers (a) Carlos, D.; Ligia, L.; Lorenzo, E.; Florencio, E. H. J. Comput. Aided. Mol. Des. 2015, 29,143. (b) Meihua, S.; Tom, G. D.Org. Lett., 2008, 10, 3367. (c) Elguero, J.; Katritzky, A. R.; Denisko, O. V. Adv. Heterocycl. Chem. 2000, 76, 1.
- Claudio C. Silveira, Samuel R. Mendes, Lucas Wolf, Guilherme M. Martins; Tetrahedron Letters2010, 51, 2014–2016.
CrossRef

This work is licensed under a Creative Commons Attribution 4.0 International License.









