Biosorption of Methylene Blue from Aqueous Solution by Coconut (Cocos Nucifera) Shell-Derived Activated Carbon-Chitosan Composite
Precious Caree V. Regunton1, Derick Erl P. Sumalapao2,3,4 and Nelson R. Villarante1
and Nelson R. Villarante1
1Department of Physical Sciences and Mathematics, College of Arts and Sciences, University of the Philippines Manila, Manila, Philippines.
2Department of Biology, College of Science, De La Salle University, Manila, Philippines.
3Mathematics Area, School of Multidisciplinary Studies, De La Salle-College of Saint Benilde, Manila, Philippines.
4Department of Medical Microbiology, College of Public Health, University of the Philippines Manila, Manila, Philippines.
Corresponding Author E-mail: derick.sumalapao@dlsu.edu.ph
DOI : http://dx.doi.org/10.13005/ojc/340113
Charcoal produced from coconut shells through the retort method was consequently activated using CaCl2. The activated carbon-chitosan composite was prepared by coating the activated carbon with chitosan. Batch experiments with methylene blue as adsorbate were conducted under varying pH, contact time, initial dye concentration, adsorbent dosage, and temperature. The effects of these factors were investigated using batch adsorption studies with optimal conditions identified at pH of 5, 30 min contact time, 10 mg L-1 initial dye concentration, 9 g L-1 adsorbent dosage, and 25 °C adsorption temperature. Results of adsorption experiments showed that the composite had better removal efficiency compared to activated carbon.
KEYWORDS:Methylene Blue; Biosorption; Cocos Nucifera; Carbon-Chitosan Composite; Retort Method; Biocharcoal; Coconut Shell
Download this article as:| Copy the following to cite this article: Regunton P. C. V, Sumalapao D. E. P, Villarante N. R. Biosorption of Methylene Blue from Aqueous Solution by Coconut (Cocos Nucifera) Shell-Derived Activated Carbon-Chitosan Composite. Orient J Chem 2018;34(1). |
| Copy the following to cite this URL: Regunton P. C. V, Sumalapao D. E. P, Villarante N. R. Biosorption of Methylene Blue from Aqueous Solution by Coconut (Cocos Nucifera) Shell-Derived Activated Carbon-Chitosan Composite. Orient J Chem 2018;34(1). Available from: http://www.orientjchem.org/?p=41826 |
Introduction
Anthropogenic activities and rapid industrialization have contributed to the worsening of environmental conditions by generating huge quantities of pollutants that are most often than not discharged into the nearest water sources. Among these contaminants which include toxic heavy metals, metalloids, radionuclides and organic compounds, dyes are of serious concern because of their hazardous compound content, suspended solids, high biological and chemical oxygen demands, and high visibility even at very low concentrations. The complex aromatic structures of dyes account for their non-biodegradability and recalcitrant nature, as exhibited by their stability against sunlight, temperature, oxidizing agents, and microbial attack1. Dye pollution poses a threat to humans’ health and ecological equilibrium due to its mutagenic, carcinogenic, and teratogenic effects upon inhalation or dermal contact, and its inhibition of sunlight penetration that may consequently lead to the reduction of photosynthetic activities of aquatic organisms2.
Among the various types of chemical and physical methods developed for the treatment of effluents, adsorption processes utilizing activated carbon are preferred due to its ease of application with good efficiency. However, commercial activated carbon is very expensive and non-renewable, which limits its practical utility especially for a large-scale setting. To address these drawbacks, production of activated carbon from cheap and renewable precursors, which are mainly agricultural by-products and wastes like coconut husks3 and coconut shells4, have been investigated. Another solution is by coating the activated carbon with a material that is not only less expensive but would also enhance its adsorption capacity5. Chitosan, with its high content of amino and hydroxyl functional groups and which is produced by alkaline N-deacetylation of chitin, exhibits high adsorption potential for a wide range of molecules such as phenolic compounds, dyes, and metal ions6. The use of biomass and biomaterials for separation of dissolved or suspended target substances from solutions is generally referred to as biosorption, a physico-chemical and metabolically-independent process that has been gaining immense interest as an alternative to conventional techniques. Hence, this study prepared coconut shell-derived activated carbon-chitosan composite as a potential biosorbent material for the removal of methylene blue in an aqueous solution by adsorption process. Moreover, the influence of pH, contact time, dye concentration, adsorbent dosage, and temperature on the adsorption process were also determined. This study also compared the removal efficiency of coconut shell-derived carbon-chitosan composite with the activated biocharcoal in the removal of methylene blue. Conversion of the widely-available agricultural wastes such as coconut shells to biocharcoal was done using a simple and low-cost method that can be adapted globally.
Materials and Methods
Materials and Reagents
Activated carbon was prepared from coconut shells obtained from a local market in Laguna, Philippines. Calcium chloride (CaCl2) was purchased from Qualikems Fine Chem Pvt. Ltd., India. Commercial chitosan (75% degree of deacetylation) was purchased from Sigma, Singapore. Pure methylene blue (C16H18ClN3S) was obtained from HiMedia, India and was used to prepare a stock solution of concentration 50 mg L-1. Serial dilutions were performed to prepare lower concentrations of the dye. The λmax of methylene blue and the absorbance of the dye solutions were determined using a Jenway 7315 spectrophotometer. Other reagents including HCl, 99% sodium hydroxide (NaOH) were obtained from RCI Labscan and oxalic acid (C2H2O4•2H2O) from HiMedia Laboratories Ltd., Mumbai. All solutions were prepared with deionized water, which was obtained from a local supplier. The pH of the solutions was adjusted with 0.1 M HCl and 0.1 M NaOH and was measured using a Sartorius Docu-pH+ meter.
Preparation of Coconut Shell Biocharcoal
Dry coconut shells were washed several times with tap water to remove dust and other soluble impurities. These were then crushed into small pieces, washed with deionized water, and oven-dried. Charcoal production was performed using the retort method7. An empty tin can was filled with the dried coconut shells and sealed with its lid, with few small holes drilled on the bottom part. The carbonization process was performed for 4-6 h by placing the can, surrounded with wood and paper materials, inside a bigger tin can with holes as vents and subsequently ignited from above. The thoroughly carbonized material was segregated and used in the subsequent experiments.
Activation Process
The charcoals were pulverized, sieved to 60-mesh, and soaked in 25% solution of CaCl2 for 24 h, and then rinsed thoroughly with deionized water and dried in an oven at 100ºC until constant weight7,8.
Preparation of Activated Carbon-Chitosan Composite
The composite was prepared by coating the activated carbon with chitosan9,10. Twenty-five grams (25 g) chitosan was added to 1 L of 0.4 M oxalic acid solution under continuous stirring at 45-50°C to facilitate formation of gel10. About 50 g of the activated carbon was slowly added to chitosan gel and stirred for 16 h, keeping the temperature at 45-50°C. The composite was then prepared by dropwise addition of the activated carbon–chitosan gel mixture11 into a 1 M NaOH bath, and washed several times with deionized water to a neutral pH9. It was dried in an oven at 100°C until constant weight. A grinder was then used to reduce the size of the activated carbon-chitosan composite and particle size of 40-mesh (0.400 µm) size was used in the adsorption study.
Batch Adsorption Studies
The effects of various reaction parameters such as pH, contact time, dye concentration, adsorbent dosage, and temperature on the adsorption process were evaluated. The flasks were kept closed to avoid fluctuation of pH. Adsorbent was separated from the solution via centrifugation. The blank for spectrophotometric determination consisted of deionized water. The experiments were performed in triplicates and the mean values were used for succeeding calculations. The same conditions were also applied to an adsorption system utilizing activated carbon to compare the efficiency of the two adsorbents. The dye uptake was computed using Equation 1, whereas the dye removal efficiency, indicated by Removal (%), was calculated using Equation 2.

where:
qe = biosorption capacity at equilibrium (mg g-1)
C0 = initial dye concentration (mg L-1)
Ce = equilibrium dye concentration (mg L-1)
V = volume of the solution (L)
M = adsorbent dosage (g)
Effect of pH
One gram per liter (1 g L-1) adsorbent was added to 20 mL methylene blue dye solution of concentration 10 mg L-1 at different pH values (4, 5, 6, 7, and 8) and temperature of 30°C. The solution was agitated at 150 rpm for 30 min.
Effect of Contact Time
One gram per liter (1 g L-1) of the adsorbent was added to 20 mL methylene blue dye solution of concentration 10 mg L-1 at optimum pH and temperature of 30°C. The solution was agitated at constant speed (150 rpm) for different time intervals (15, 30, 45, 60, and 75 min).
Effect of Initial Dye Concentration
One gram per liter (1 g L-1) of the adsorbent was added to 20 mL methylene blue dye solution of different concentrations (2, 4, 6, 8, and 10 mg L-1) at optimum pH and temperature of 30°C. The solution was agitated at 150 rpm for optimum contact time.
Effect of Adsorbent Dosage
Various amounts of the adsorbent (1, 2, 3, 4, 5, 6, 7, 8, and 9 g L-1) were added to 20 mL methylene blue dye solution at optimum concentration, pH, and temperature of 30°C. The solution was agitated at 150 rpm for optimum contact time.
Effect of Temperature
One gram per liter (1 g L-1) of the adsorbent was added to 20 mL methylene blue dye solution at optimum concentration, pH, and different temperature (25, 40, 50, and 60°C). The solution was agitated at 150 rpm for optimum time.
Results and Discussion
Batch Adsorption Studies
Solid-liquid adsorption system is assessed usually employing either the batch adsorption tests or the dynamic continuous-flow adsorption tests12, the former of which was used in this study. In batch adsorption studies, which are relatively cheap and simple procedures, the different biosorption parameters can be easily adjusted, monitored, and optimized. Prior to the experiments, a wavelength scan of an aqueous solution of methylene blue was done to determine its λmax. The maximum absorbance of 1.847 occurred at a wavelength of 665 nm (Figure 1), and this wavelength was used in further absorbance measurements.
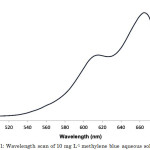 |
Figure 1: Wavelength scan of 10 mg L-1 methylene blue aqueous solution. |
Effect of pH
The pH of the dye solution plays a key role in the adsorption process by influencing the surface charge of the biosorbent, degree of ionization of the compounds present13, dissociation of functional groups, and the solution dye chemistry. Hydrogen and hydroxyl ions are adsorbed quite strongly and therefore, the adsorption of other ions is affected by the pH of the solution14,15. Chitosan, which is a weak base and is relatively insoluble in water and in some organic solvents, is highly soluble in dilute aqueous acidic solutions (pH < 6.5)12. Chitosan has pKa values ranging from 6.5 to 6.7 depending on the degree of deacetylation, ionic strength, and charge neutralization of the amine groups16. Moreover, chitosan forms gels at lower pH and precipitates in alkaline solution or with polyanions12.
The effect of pH on the adsorption of methylene blue by activated carbon-chitosan composite was determined by varying the pH from 4 to 8 (Figure 2). Highly acidic conditions were not included in the study because of the apparent disintegration of the composite. This indicates dissociation of the chitosan from the activated carbon since the former is soluble in acidic media. Highly alkaline conditions were also excluded because of the formation of hydrogen bonds between the amine groups of chitosan, which caused a decreased in the number and diameter of the pores, thus effectively decreasing the dye uptake17. In this study, the maximum uptake was observed at pH 5 (Figure 2).
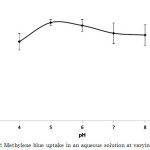 |
Figure 2: Methylene blue uptake in an aqueous solution at varying pH values. |
Effect of Contact Time
In adsorption process, contact time is one of the important parameters to monitor because it provides pertinent information on the adsorption capacity, desorption efficiency, and regeneration potential of the adsorbent12. Rapid increase in dye uptake was observed in the first 30 min, and subsequently decreased thereafter (Figure 3). These changes in the dye uptake may be due to, initially, the adsorbent sites were all available for binding of the adsorbate molecules5,18. During the process, the surface of the activated carbon-chitosan composite was saturated with methylene blue, thus preventing the binding of additional dye molecules. At 30 min, saturation of the sites was observed and hence, equilibrium was attained. However, analyses beyond this period showed decreasing dye uptake which can be attributed to the desorption of the dye adsorbate from the composite. This gives a good opportunity for the regeneration and recycling of the composite for another batch of adsorption. The dye uptake at 30-min contact time was 3.357 mg g-1 (Figure 3).
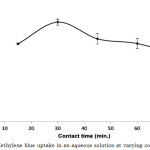 |
Figure 3: Methylene blue uptake in an aqueous solution at varying contact time. |
Effect of Initial Dye Concentration
In an adsorption process, the initial dye concentration reflects the amount of dye molecules available for binding. Generally, at constant adsorbent dosage, dye uptake increases with increase in dye concentration due to mass transfer phenomenon. The dye removal efficiency, on the other hand, decreased with an increase in the initial dye concentration, which can be attributed to the saturation of the adsorption sites5,18. In this study, methylene blue-composite system obeyed these general trends (Figure 4).
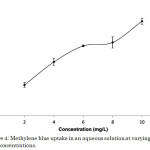 |
Figure 4: Methylene blue uptake in an aqueous solution at varying initial dye concentrations. |
Methylene blue molecules tend to diffuse from the solvent (water) towards the surface of the activated carbon-chitosan composite because of concentration gradient. The driving force of this factor increased with an increase in the initial dye concentration, and hence led to greater dye uptake (Figure 4). The maximum dye uptake of 3.357 mg g-1 was observed at 10 mg L-1 of methylene blue solution corresponding to 33.57% removal efficiency. However, the residual concentration of dye molecules, that is the amount of methylene blue molecules not taken up by the adsorbent, increased with higher initial concentration. This explains the decrease in the dye removal efficiency whose maximum value of 54.54% corresponding to a dye uptake of 1.091 mg g-1 was observed at 2 mg L-1 methylene blue solution (Figure 5). This point is made evident in Table 1, where Ce represents the concentration of methylene blue solution after adsorption.
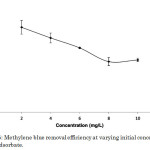 |
Figure 5: Methylene blue removal efficiency at varying initial concentrations of dye adsorbate. |
Table 1: Comparison of the dye uptake and dye removal efficiency of varying initial concentrations of methylene blue solutions
|
Initial Concentration (mg L-1) |
Ce (mg L-1) |
Dye Uptake (mg g-1) |
Dye Removal Efficiency (%) |
|
2 |
0.909 |
1.091 |
54.54 |
|
4 |
2.091 |
1.909 |
47.72 |
|
6 |
3.520 |
2.480 |
41.32 |
|
8 |
5.398 |
2.602 |
32.53 |
|
10 |
6.643 |
3.357 |
33.57 |
Effect of Adsorbent Dosage
Adsorbent dosage is an important parameter since it determines the capacity of an adsorbent for a given amount of adsorbate at specified conditions. Increasing the adsorbent dosage generally increased the removal efficiency due to increased adsorbent surface and availability of more adsorption sites5,14,18. However, if the adsorption capacity was expressed as milligram adsorbed per gram of material, as in the case of dye uptake, a decrease was observed with increasing dosage due to the overlapping or aggregation of adsorption sites12. In this study, these trends were followed by the methylene blue-composite adsorption system (Figure 6). Comparison of the dye uptake and dye removal efficiency at varying adsorbent dosages is shown in Table 2. The optimum adsorbent dosage was 9 g L-1 with a dye uptake of 1.003 mg g-1 corresponding to 90.61% removal efficiency (Figure 7). Despite the low dye uptake per gram of the material, it must be noted that the presence of more amount of adsorbate resulted in a very high overall efficiency.
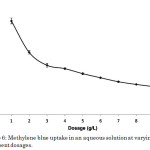 |
Figure 6: Methylene blue uptake in an aqueous solution at varying adsorbent dosages. |
Table 2: Comparison of the dye uptake and dye removal efficiency of the different adsorbent dosages.
|
Adsorbent Dosage (g L-1) |
Ce (mg L-1) |
Dye Uptake (mg g-1) |
Dye Removal Efficiency (%) |
|
1 |
6.643 |
3.357 |
33.57 |
|
2 |
5.540 |
2.230 |
44.60 |
|
3 |
4.695 |
1.768 |
53.05 |
|
4 |
3.405 |
1.649 |
65.95 |
|
5 |
2.651 |
1.470 |
73.49 |
|
6 |
2.068 |
1.322 |
79.32 |
|
7 |
1.740 |
1.180 |
82.60 |
|
8 |
1.331 |
1.084 |
86.69 |
|
9 |
0.939 |
1.003 |
90.61 |
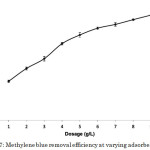 |
Figure 7: Methylene blue removal efficiency at varying adsorbent dosages. |
Effect of Temperature
Temperature affects the adsorption capacity of the adsorbent. An increase in the adsorption capacity with increasing temperature indicates that the adsorption process is endothermic19, the mobility of the adsorbate molecules as well as the number of active sites for adsorption both increase at higher temperatures. On the other hand, a decrease in the adsorption capacity with increasing temperature indicates that the adsorption process is exothermic19. This may be attributed to higher temperatures decreasing the adsorptive forces between the adsorbate species and the active sites on the adsorbent surface resulting to a decreased adsorption capacity. The adsorption of methylene blue onto keratin nanofibrous membranes20 and the adsorption of acid red 88 dye onto bio-silica-chitosan composite21,22 also showed this trend.
Increasing the temperature from 25 to 60°C resulted in a decrease in dye uptake (Figure 8). Hence, the adsorption of methylene blue to the surface of the activated carbon-chitosan composite is an exothermic process. Generally, adsorption of organic compounds including dyes is an exothermic process, thus increasing temperature weakens the binding interaction between the dye adsorbate and the active sites of the adsorbent12. In this study, the most favorable temperature was observed at 25°C with a dye uptake of 4.722 mg g-1 corresponding to 47.22% removal efficiency.
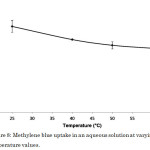 |
Figure 8: Methylene blue uptake in an aqueous solution at varying temperature values. Click here to View figure |
The favorable conditions determined in the batch studies were applied to compare the dye removal efficiency of the composite with that of activated carbon. The activated carbon and composite had dye uptakes of 0.966 mg g-1 and 1.009 mg g-1 corresponding to dye removal efficiencies of 86.94% and 90.77%, respectively. This means that the composite was more efficient than the activated carbon. Since coating the activated carbon with chitosan up to five times had increased the thermal stability of the adsorbent and decreased the crystalline nature of the chitosan enhancing its adsorption efficiency on the removal of heavy metals23.
Conclusion
The activated carbon-chitosan composite was successfully prepared from coconut shells, CaCl2, and commercial chitosan. The effects of the different factors were investigated using batch studies and the most favorable conditions were determined at pH of 5, 30 min contact time, 10 mg L-1 initial dye concentration, 9 g L-1 adsorbent dosage, and 25°C adsorption temperature. Comparison with activated carbon showed that the composite had a better removal efficiency of 90.77%.
References
- Akar, S.; Gorgulu, A.; Akar, T.; Celik, S. Decolorization of reactive blue 48 contaminated solutions by Capsicum annuum seeds: Batch and continuous mode biosorption applications. Chemical Engineering Journal. 2011, 125-133.
CrossRef - Abdallah, R.; Taha, S. Adsorption of methylene blue from aqueous solution by nonviable Aspergillus fumigatus. Chemical Engineering Journal. 2012, 69-76.
CrossRef - Tan, I.; Ahmad, A.; Hameed, B. Enhancement of basic dye adsorption uptake from aqueous solutions using chemically modified oil palm shell activated carbon. Colloids and Surfaces. 2008, 88-96.
CrossRef - Ashish, S.; Aniruddha, M.; Prathmesh, S.; Dattatraya, P.; Prakash, R.; Mansing, A.; Sanjay, K. Removal of Bi (III) with adsorption technique using coconut shell activated carbon. Chinese Journal of Chemical Engineering. 2012, 768-775.
- Villarante, N.R.; Bautista, A.P.R., Sumalapao, D.E.P. Batch adsorption study and kinetic profile of Cr(VI) using lumbang (Aleurites moluccana)-derived activated carbon-chitosan composite crosslinked with epichlorohydrin. Orient. J. Chem. 2017, 33(3), 1111-1119.
CrossRef - Zhang, J.; Liu, F. Adsorption of natural organic matter onto a composite adsorbent prepared with chitosan and powdered activated carbon. Desalination and Water Treatment. 2010.
CrossRef - Cobb, A.; Warms, M.; Maurer, E.; Chiesa, S. Low-tech coconut shell activated charcoal production. International Journal for Service Learning in Engineering. 2012, 93-104.
- Renault, F. Cationized starch based material as a new ion exchanger adsorbent for the removal of C.I. Acid Blue 25 from aqueous solutions. Bioresource Technology. 2008.
CrossRef - Hydari, S.; Sharififard, H.; Nabavinia, M.; Parvizi, M. A comparative investigation on removal performances of commercial activated carbon, chitosan biosorbent and chitosan/activated carbon composite for cadmium. Chemical Engineering Journal. 2012, 276-282.
CrossRef - Sharififard, H.; Soleimani, M.; Ashtiani, F.Z. Evaluation of activated carbon and biopolymer modified activated carbon performance for palladium and platinum removal. Journal of the Taiwan Institute of Chemical Engineers. 2012.
- Sharififard, H.; Ashtiani, F.Z.; Soleimani, M. Adsorption of palladium and platinum from aqueous solutions by chitosan and activated carbon coated with chitosan: adsorption of palladium and platinum ions from aqueous solutions. Asia Pacific Journal of Chemical Engineering. 2012.
- Crini, G.; Badot, P. Application of chitosan, a natural polysaccharide, for dye removal from aqueous solutions by adsorption processes using batch studies: A review of recent literature. Progress in Polymer Science. 2008, 399-447.
CrossRef - Greluk, M. Efficient removal of Acid Orange 7 dye from water using the strongly basic anion exchange resin Amberlite IRA958. Desalination. 2011.
CrossRef - Barka, N.; Abdennouri, M.; Makhfouk, M. Removal of methylene blue and Eriochrome Black T from aqueous solutions by biosorption on Scolymus hispanicus L.: Kinetics, equilibrium and thermodynamics. Journal of the Taiwan Institute of Chemical Engineers. 2011, 320-326.
- Doke, K. M.; Yusufi, M; Joseph, R.D.; Khan, E.M. Comparative adsorption of crystal violet and congo red onto ZnCl2 activated carbon. Journal of Dispersion Science and Technology. 2016.
CrossRef - Guibal, E. Heterogeneous catalysis on chitosan based materials: a review. Progress in Polymer Science. 2005.
CrossRef - Ngah, W.; Teong, L.; Hanafiah, M. Adsorption of dyes and heavy metal ions by chitosan composites: A review. Carbohydrate Polymers. 2011, 1446-1456.
CrossRef - Sumalapao, D.E.P.; Distor, J.R.; Ditan, I.D.; Domingo, N.T.S.; Dy, L.F.; Villarante, N.R. Biosorption kinetic models on the removal of congo red onto unripe calamansi (Citrus microcarpa) peels. Orient. J. Chem. 2016, 32(6), 2889-2900.
CrossRef - Yagub, M.; Sen, T.; Afroze, S.; Ang, H. Dye and its removal from aqueous solution by adsorption: A review. Advances in Colloid and Interface Science. 2014, 1-13.
CrossRef - Aluigi, A.; Rombaldoni, F.; Tonetti, C.; Jannoke, L. Study of methylene blue adsorption on keratin nanofibrous membranes. Journal of Hazardous Materials. 2014, 156-165.
CrossRef - Soltani, R.; Khatee, A.; Safari, M.; Joo, S. Preparation of bio-silica/chitosan nanocomposite for adsorption of textile dye in aqueous solutions. International Biodeterioration & Biodegradation. 2013, 383-391.
CrossRef - Noorimotlagh, Z.; Darvishi Cheshmeh Soltani, R.; Khataee, A.R.; Shahriyar, S.; Nourmoradi, H. Adsorption of a textile dye in aqueous phase using mesoporous activated carbon prepared from Iranian milk vetch. Journal of the Taiwan Institute of Chemical Engineers. 2014.
- Soundarrajan, M.; Gomathi, T.; Sudha, P. Understanding the adsorption efficiency of chitosan coated carbon on heavy metal removal. International Journal of Scientific and Research Publications. 2013, 1-10.

This work is licensed under a Creative Commons Attribution-NonCommercial-ShareAlike 4.0 International License.

This work is licensed under a Creative Commons Attribution 4.0 International License.









