Thermodynamic Properties of Complex Ferrite BiCa3Fe5O12
Mukhametkali Musagalievich Matayev1, Moldir Rashidovna Abdraimova1, Zhanar Yliasovna Tursinova1 and Assel Taupykovna Kezdikbaeva2
1Kazakh State Women`s Teacher Training University, 050000, Aiteke bi, 99, Almaty, Kazakhstan.
2Karaganda State University named by Y.A.Buketov, Universitetskaya 28, Karagandy, Kazakhstan.
Corresponding Author E-mail: matayevm@bk.ru
DOI : http://dx.doi.org/10.13005/ojc/330666
The compound BiCa3Fe5O12 was synthesized by the solid-phase reaction method. For the first time, the thermodynamic properties were calculated using semiempirical methods: Standard enthalpy of formation, Н0298,Standard heat capacity, С0Р, 298,Standard Gibbs energy, G0298andStandard entropy of formation, S0298, coefficients in the equation of the temperature dependence of the heat capacityCp(T). The temperature dependence of the specific heat was studied by dynamic calorimeter. Thus, in the temperature interval 298.15-673 K, the specific heats of the complex ferrite BiCa3Fe5O12 were experimentally determined, and the polynomial equation of the temperature dependence of the heat capacity was derived.The comparative analysis of the experimental and calculated values of the specific heat wascarried out.
KEYWORDS:Oxides; Ferrites; Heat Capacity; Calorimeter
Download this article as:| Copy the following to cite this article: Matayev M. M, Abdraimova M. R, Tursinova Z. Y, Kezdikbaeva A. T. Thermodynamic Properties of Complex Ferrite BiCa3Fe5O12. Orient J Chem 2017;33(6). |
| Copy the following to cite this URL: Matayev M. M, Abdraimova M. R, Tursinova Z. Y, Kezdikbaeva A. T. Thermodynamic Properties of Complex Ferrite BiCa3Fe5O12. Orient J Chem 2017;33(6). Available from: http://www.orientjchem.org/?p=39901 |
Introduction
Investigation of the physico-chemical properties of ferrites formedin the Bi2O3-MeIIO-Fe2O3 (MeII-alkaline-land metal) systems is of definite scientific and practical interest for the directed synthesis of compounds with specified properties.The analysis of literature data shows that the most studied of ferrites arethe BiFeO3orthoferrites, so-called multiferroics, which possess both electric polarization and magnetic ordering, recently there has been a significant increase in interest in these classes of compounds, their connection with promising applications as a working medium in devices of storage and information processing. On the basis of the most famous multiferroics, a wide search for new materials with ferroelectric properties is carried out by specific electronic and magnetic structures. The mixed result is based on bismuth ferrite which often combines ferroelectric and weakly ferromagnetic properties with the dominant antiferromagnetic ordering [1-2].Experimental studies, as a rule, are time-consuming, expensive and do not always yield reliable results. An alternative source of obtaining new information and revising the availableis the use of computational methods, which are based on the idea of thermodynamic similarity connecting the physicochemical properties of the system with its composition [3].
In this paper, semiempirical calculations of the thermodynamic properties and the temperature dependence of the heat capacity of complex ferrite BiCa3Fe5O12werecarried out. The thermodynamic characteristics obtained are compared with the experimental data.
Experimental part
The complex ferrite was obtained by the solid-phase synthesis method in an alundum crucible from pre-annealed and thoroughly grindedfor 56 hours powders Fe2O3 (hp), CaCO3 (bp) and Bi2O3 (bp).The composition of the obtained compound was confirmed by X-ray diffraction analysis using a RigakuMinilex 600 diffractometer.
The heat capacity of ferrites was studied by the method of dynamic calorimeter on a serial IT-S-400 instrument in the temperature range 298-673 K. The experiments were carried out in a monotonic regime, close to linear heating of the sample with an average rate of about 0.1 K per second. The maximum error in measuring the heat capacity on the S-400 IT device, according to the passport data, is ± 10% [9].
Results
The compound BiCa3Fe5O12 was synthesized by the solid-phase reaction method and for the first time, the thermodynamic properties were calculated using semiempirical methods.All the calculated properties of the compound are shown in Table 1.
Table 1: Thermodynamic properties of BiCa3Fe5O12 obtainedby semiempirical methods
|
BiСа3Fe5O12 |
|
|
ΔH0298(ox), kJ / mol |
-192.582±61.74 |
|
ΔH0298, kJ / mol |
-4443.5 ± 61.74 |
|
S0298, kJ / mol |
371.6±8 |
|
С0Р,298, kJ / mol |
397.95 ±88.7 |
|
G0298, kJ / mol |
– 3,684.94 |
|
а |
82 |
|
b, 10-3 |
775· 103T |
|
c, 105 |
122 ·10-5T-2 |
Discussions
Semiempirical calculation of thermodynamic properties:Information on the properties of simple oxides and Bi2O3 and Fe2O3, CaO was taken in [3].
Standard Enthalpy of Formation, Н0298
The standard enthalpy of formation is calculated by the formula used to estimate the heat of the compounds, which can be represented as pseudobinary or pseudotraic [4]
H° 298 (j) = Σ ni ΔH° 298 (i) + ΔH° 298(ox),
where ΔH0 298, ni is the standard heat of formation and the number of moles of the i-th compound in the j-th complex; ΔH0298 (ox) – SEO of a compound of simpler compounds.
In accordance with this formula, we can write
H° 298 (BiCa3 Fe5O12) = 0,5. ΔH° 298 (Bi2O3) + 3. ΔH° 298 (CaO) + 2,5. ΔH° 298 (Fe2O3) + ΔH° 298(ox)
To estimate the value of ΔH0298 (ox) complex oxides, the empirical dependence was used [4].
H° 298(ox) ≈ (-16,0485) ± 5,145.mo,
where m0 is the number of oxygen atoms in the compound formula.
As a result, we received:
H° 298(ox) ≈ 192,582 ± 61,74kJ/ mol
and
H° 298 (BiCa3 Fe5O12) ≈ 4443,5+61,74kJ/ mol
Standard entropy of formation, S0298
The standard entropy of the formation of S0298 is calculated by three methods: additively by the Kopp-Neumann rule using S0298 simple oxides [5], the Latimer method [4] and the Kumok increments method [4]. The additive method for calculating S0298 is based on the addition of S0298 simple oxides that make up the compound in the molar ratio:
S° 298 = n. S° 298 (Bi2 O3) +m. S°298 (CaO)+ r. S° 298(Fe2O3)
S° 298 =(BiCa3 Fe5O12)=419,2J (mol.K)
The Kumok increments method assumes the computation by equation
S° 298 =SK.nk+ Sa.na+Sl.nl,
where Sk and Sa, Sl are increments of cations and anions, respectively (values are taken in [4]), nk and na, nl is the number of cation and anion compounds making up a compound.
For BiСa3Fe5O12 S0298, the Kumok increments method was 427.2 J/(mol∙K). Using the Latimer method, it was 268.4 J/(mol∙K). The average arithmetic value of the standard entropy of formation is 371.6 ± 8J/(Kmol).
Standard heat Capacity, С0Р, 298
The standard heat capacity was calculated according to the Kopp-Neumann rule [5]:
C° P, 298=n. C° P, 298 (CaO) +k. C° P,298(Bi2O3)+1. C°P, 298 (Fe2O3)
and equaled 353.6 J/(mol∙K), according to the dependence [5].
As per the Kumok increments method:
C °P, 298 = C° P, 298 K.nk + C° P,298a.nc,
where C0 p, 298K and C0 p, 298a, C0 p, 298a ∙ nc cation and anion increments, respectively, n·k and n·a, n·c is the number of cation and anion compounds making up the compound, the heat capacity is equal to 442.3J/(mol∙K).
The average value of 397.95 ± 88.7 is listed in Table 1.
Standard Gibbs Energy, G0298
The standard Gibbs energy is calculated by the method of ion increments. Standard Gibbs energy of a solid salt according to this method is calculated according to the following formula:
![]()
Where Mn+ is a cation, Xm– is an anion, m, n– are indices of cations and anions. Using the alkaline, alkaline earth, transition, and rare-earth metal cations in standard aqueous solution and anions (ΔG0298), the standard Gibbs energies of the salts of the s, d, f elements in the solid state can be calculated [6-7].
Received
![]()
Temperature Dependence of the Heat Capacity, Cp (T)
The temperature dependence of the heat capacity was calculated in the following ways: an additive method using data on simple oxides [5].
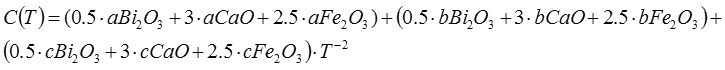
Received
C (T) = 82 +775.103 T-122.10-5 T-2
Experimental Findingof Heat Capacity
Calorimetric methods for studying the thermodynamic properties of solids were used to measure the dependence of the heat capacity of solids on temperature and the change in energy during cation exchange. On anIT-S-400 calorimeter at the temperature interval 298.15-650 K, the comparative heat capacities of the synthesized BiСa3Fe5O12 compounds were foundexperimentally. Figure 1 shows that the heat capacity of substances increases with increasing temperature. One of the reasons for the increase in heat capacity with increasing temperature is the energy expenditure on the formation of a vacancy upon the transition of the nodes of crystal lattices to intermediate sites [8-9].
For BiСa3Fe5O12, the temperature dependence of the heat capacity has the form shown in Fig. 1.
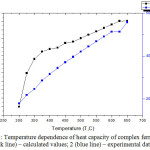 |
Figure 1: Temperature dependence of heat capacity of complex ferrite: 1 (black line) – calculated values; 2 (blue line) – experimental data; Click here to View figure |
Conclusion
The results of calculations of the thermodynamic properties of complex ferrite, carried out using semiempirical methods, are in satisfactory agreement with those values that could be obtained experimentally. Thus, for the first time in the temperature range 298.15-673 K, the specific heats of the complex ferrite BiСa3Fe5O12 were experimentally found, and the polynomial equation of the temperature dependence of the heat capacity was derived. A comparative analysis of the experimental and calculated values of the specific heat wascarried out.
Acknowledgment
The article was prepared with the financial support of the grant of the Ministry of Education and Science of the Republic of Kazakhstan No. 3288 / GF4 “Synthesis and physical and chemical studies of new generation multifunctional magnetic materials” (under contract No. 249 of March6, 2017)
References
- Lisnevskaya I.A., Bobrova T.G., Lupeiko M.R., Agamirzoeva K.V. and Myagkaya, Y3Fe5O12 / Na, Bi, Sr-doped PZT particulate magneto electric composites. I.V. Faculty of Chemistry, Southern Federal University. Journal of Magnetism and Magnetic Materials; 2016, 405: 62-65.
CrossRef - Ramesh R. and Spaldin, N. A., “Multiferroics: progress and prospects in thin films”. Nature Materials; 2007, 6: 21.
CrossRef - Belousova N.V. and Arkhipova E.O., Thermodynamic properties of bismuth pyrostannate. Polzunovsky Herald; 2009, 3: 56-59
- Kim H. W., Shim S. H. and Lee J. W., Chemical Physics Letters; 2008, 456: 193-197.
CrossRef - Evans I. R., Judith A.K. Howard and John S.O. Evans., J. Mater.Chem; 2003, 13: 2098-2103.
CrossRef - Moiseev G.K., Vatolin N.A., Marshuk L.A. and Ilyinikh N.I., Temperature dependences of the reduced Gibbs energy of some inorganic substances. Ekaterinburg: UrB RAS. 1997, pp. 230
- Moiseev G.K. and Vatolin N.A., Some regularities of change and methods for calculating the thermochemical properties of inorganic compounds. Ekaterinburg: UrB RAS, 2001, pp. 135.
- Kasenov B.K., Aldabergenov M.K. and Pashkinin A.S., Thermodinamic methods in chemistry and metallurgy. Almaty: “Rauan”, “Demeu”, 1994, pp. 127.
- Matayev, M.M.,Myrzahmetova, N.O., Nuketayeva, D. Zh., Zhumanova, A., Kuanysheva, Zh.K., Nurbekova, M.A. and Abdraimova, M.R., Heat Capacity and Thermodynamic Functions of Complex Magnates and Ferrites in the Temperature Range 298.15-673K. Middle East journal of Scientific.-Research (MEJSR); 2013, 4 (1): 5-8

This work is licensed under a Creative Commons Attribution 4.0 International License.









