The Influence of type of Functional Groups on the Adsorption Selectivity of Ionic Imprinted Polymer (Iip) Fe
Muhammad Cholid Djunaidi , Retno Ariadi Lusiana, Pardoyo and Meta Dian Arini
, Retno Ariadi Lusiana, Pardoyo and Meta Dian Arini
Department of Chemistry, Faculty of Science and Mathematics Diponegoro University, in Semarang, Indonesia.
Corresponding Author E-mail: choliddjunaidi@live.undip.ac.id
DOI : http://dx.doi.org/10.13005/ojc/330635
The selectivity of Ionic Imprinted Polymer (IIP) Fe metal ion using functional polymers of eugenol derivatives was undertaken through the adsorption process. The forms of functional polymers were polyeugenol (PE), acetic polieugenoxy acid (PA) and poly tiophen-2-methyl-2-eugenoxy acetate (PFMEA). The purposes of this research were to know the influence of functional groups on the functional polymer of eugenol derivatives to the adsorption selectivity of IIP involving imprint cavity. The method in this research is the Ion Imprinted polymer (IIP) produced a metal ion imprint bound with the polymer, locked by Ethylene dimethacrylate (EDMA) then removed from the polymer to produce imprint selective to the ion imprinted. The adsorption selectivity of Fe metal ions in PA was higher than PE and PFMEA. Analysis of surface area shows that IIP PA has larger surface area compared with NIP. The adsorption selectivity of Fe was higher on Fe-Cr than Fe-Cd and Fe-Pb.
KEYWORDS:Polyeugenol; Ionic Imprinted Polymer (IIP); Ethylene Dimetachrylate; Selective Adsorption
Download this article as:| Copy the following to cite this article: Djunaidi M. C, Lusiana R. A, Pardoyo P, Arini M. D. The Influence of type of Functional Groups on the Adsorption Selectivity of Ionic Imprinted Polymer (Iip) Fe. Orient J Chem 2017;33(6). |
| Copy the following to cite this URL: Djunaidi M. C, Lusiana R. A, Pardoyo P, Arini M. D. The Influence of type of Functional Groups on the Adsorption Selectivity of Ionic Imprinted Polymer (Iip) Fe. Orient J Chem 2017;33(6). Available from: http://www.orientjchem.org/?p=39717 |
Introduction
Chemiclas water polution is a problem all over the world. Heavy metals ion is one of the most dangerous because it is very toxic even in small quantities1. Although the iron metal is an essential mineral, existence of this metal in drinking water and ground water can cause a problem, such as discolourization, giving metal taste, the smell of muddiness and giving the spots on the laundry and plumbing. Iron oxide formed in tandon (reservoir) from the aeral oxidation of dissolved iron, evokes the growth of microorganisms in the water too. So, the world health organization, WHO (World Health Organization) makes a limitation iron 0,3 mg/L in drinking water2.
Development of cheap adsorbent which has high adsorption capacity is the main goal of much research. Ion imprinted polymer (IIP) is a method of printing metal into polymer, then it is released from the polymer matrix to produce imprint appropriate for ion imprinted. The advantages of IIP are high selectivity and easy prepared3.
Eugenol as the indonesia’s natural ingredient is used for the separation or the metal ion. Eugenol can also be used as a basic material for synthesis of a compound because of the presence of its three functional groups, so it is potential for the functional monomer for the selective adsorption process like Ionic Imprinted Polymer (IIP).
In this study the synthesis of IPP was undertaken using eugenol derivatives in the form of functional polymer variations; they are polyeugenol (PE), acetic polyeugenoxy acid (PA) and poly-thiohene 2-methyl-2-eugenoxy acetate (PFMEA) and cross linker agent EDMA (ethylene dimethacrylate) and AIBN initiator ((2.2′, azobis (2-metilpropionitril)) to test the selectivity adsorption for Fe metal.
Experiment
The results of the synthesis of functional polymer of eugenol derivatives were used to synthesize both IIP and NIP in which in the synthesis of IIP, it was carried out contacting template in the form of Fe ions and then crosslinked with EDMA as a corsslink agent and AIBN as an initiator. The results obtained were then released of their Fe ions using acid, acid filtrate was added KSCN as complex agent to form complex compound which can be tested using UV-Vis spectrophotometer. Meanwhile, in the synthesis of NIP has the same procedure but the contacting with Fe ion in the beginning of the process was not undertaken. The final step was the adsorbent adsorption of functional polymer contacted with a binary metal mixture for 24 hours. Then, the filtrate of adsorption process was analyzed using atomic absorbtion spectrometer (AAS).
Instruments
Laboratory glassware, analytical balance, reflux apparatus, magetic stirrer, spatula, pH meter, filter paper, UV-Vis spectrophotometer (Shimadzu), Atomic Absorption spectroscopy (AAS) (Perkin Elmer) , and Fourier Transform Infra Red (FTIR) (Nicolete Avatar 360), Scanning Electron Microscopy (SEM) (JSM-6510), Brunauer, Emmet dan Teller (BET)(Quantachrome).
Materials
Eugenol p. a, BF3diethyl ether, Ethylene dimethacrylate (EDMA), AIBN (2.2′, Azobis (2-methylpropionitrile), 2-thiophenemethanol and SOCl2 that were purchased from SIGMA Aldrich, Anhydrous Na2SO4, HCl, NaHCO3, NaOH, ClCH2COOH, Chloroform, Diethylether, KSCN, Fe(NO3)3, Cr(NO3)3 Pb(NO3)2, Cd(NO3)2 that purchased from Merck, Aquademinaralized (Bratachem)
Synthesis Materials
Synthesis of Functional Polymers
Synthesis of Polyeugenol (PE).4
5,8 gram eugenol (0,035 mol) was poured in the three-neck flask then added 1 mL BF3-diethylether. The mixture was then stirred using stirer for 4 hours and in every 1 hour 0.25 mL BF3-diethylether was added into it. The polymerization reaction occured during 12-16 hours, polymerization was stopped by adding 1 mL of methanol. The gel formed was then dissolved in chloroform and then washed with aquadest till neutral pH was achieved. The solution was then dried by adding anhydrous Na2SO4. After the solution was free of water/aquadest, the solution was evaporated at room temperature. The sediment formed was dried and weighed. The product was analyzed using FTIR.
Synthesis of Acetic Polyeugenoxy Acid (PA).5
5 gram polyeugenol (0,03 mol) was added into the boiling flask with the size 100 mL, after that, it was added 33% NaOH solution (0,825mol) as much as 17,5 mL. Then, the mixture was stirred for about 30 minutes, and added 12,5 mL of 50% chloroasetic acid solution (0,529 mol) drop by drop using pipette while stirring constantly. The mixture was heated in a water bath with temperature of 80-90oC. The heating process was carried out for 2 hours, then cooled and acidified with 6 M HCl to obtain pH 1. Subsequently it was extracted using 50 mL diethylether 3 times. Ether extracts were combined and extracted with 30 mL of 5% sodium bicarbonate b/v (0,059 mol) 3 times, then a layer of water was acidified with 6 M HCl to obtain pH 1. Moreover, filtration, drying, and weighing to the product were conducted. The result obtained was analyzed using FTIR.
Synthesis of Poly-Thiophene 2-Methyl-2-Eugenoxy Acetate (PFMEA)
3 grams PA (0,0135 mol) put in the bolling flask was added 3 mL of thionyl chloride (0,04 mol), then refluxed for 240 minutes (400C) and after that allowed to cool. The next step was adding 2,5 ml of 2-Thiophenemethanol (0,027 mol) drop by drop and then refluxing back for 240 minutes (400C). After getting cool, it was dissolved in chloroform and washed with aquadest, dried using anhydrous Na2SO4. Subsequently, filtration and evaporation of the solvent were undertaken. The results was then analyzed using FTIR.
Synthesis of IIP and NIP
Synthesis of Fe IIP
0.5 gram polyeugenol (0,003 mol) was stirred with ion Fe (III) in certain concentration for 24 hours. Then, it was filtered using a filter paper and dried in the room temperature until dry. Polyeugenol-ion Fe (III) produced of about 0,222 g was then crosslinked with 0,4 ml (5 mol) EDMA as a cross linker agent, it was then mixed with 1,67 ml chloroform and added 0,48 ml AIBN as an initiator. After that, the mixture was refluxed till the temperatures reached 110oC. The result in the form of sediment was then dried. 0,2 g of polymer sediment produced was then treated with acid for 24 hours to release Fe (III) ion and finally, ion Fe(III) IIP was generated.
The next procedure was the same as synthesis of Fe-IIP procedure, the difference lay on the functional polymers used, namely, acetic polyeugenoxy acid and thiophen of poly-2-methyl-2-eugenoxy acetate.
Synthesis of NIP
NIP was synthesized in the same way with IIP but without the binding of Fe (III) first. Characterization of IIP and NIP was carried out using FTIR.
Adsorption Experiment
50 mg adsorbent was contacted with the mixture of 10 ml ion Fe (III) (10 ppm) and 10 ppm competitor ion for 24 hours and stirred constantly. The mixture was filtered with a smooth filter paper.
The selective Adsorption Test
The selective adsorption test was undertaken on the competitive adsorption of binary aqueous consisting of Fe(III)/Cd(II), Fe(III)/Cr(III), Fe(III)/Pb(II) and then compared to NIP.
Materials Characterization
The concentration of Fe (III) and competitor metal ion in the filtrate of the adsorption experiment result was determined using Atomic Absorbtion Spectrometer (AAS). FTIR, BET and SEM were analysed for cahacterisation materials.
Results and Discussion
Syntesis of Materials
Synthesis Polyeugenol (PE), polyeugenoxy acetic acid (PA) were analised and discussed in others paper. 4-10
Synthesis of Poly-Thiophene 2- Methyl 2- Eugenoxy Acetate
Synthesis of poly-thiophene 2-methyl 2-eugenoxy ecetate is a reversible esterification reaction with high yield so that thiophene methyl eugenoxy acetate should be produced in the form of chloric acid by the addition of thionyl chloride to obtain the yield more than 80%. Then, it was added chloroform as a solvent to protect the ester formed from being hydrolyzed back. The result obtained was brownish-brown in the powder form with a distinctive odor and yield 93,17%. Further it was analyzed using FTIR to identify the character of eugenol ester.
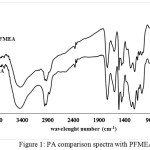 |
Figure 1: PA comparison spectra with PFMEA Click here to View figure |
The FTIR spectra in figure 1 shows that compounds analyzed have C=C bond indicated with the presence of absorption band at 1627 cm-1, Csp2-H with the absorption band at 2931 cm-1, C-C with the absorption band at 1435 cm-1, and the absorption band at 1735 cm-1 and the loss of acid C-O absorption at 1149 cm-1 replaced with the appearence of ester C-O absorption at 1072 cm-1, it can be concluded that the poly-thiophene-2-methyl-2-eugenoxy acetate was formed.
Synthesis of IIP
In the synthesis of IIP, each functional polymer was contacted with 50 ppm Fe (III) ion (pH 3) and stirred for 24 hours. Then the result was filtered using filter paper and dried. Fe-functional plymer produced was cross-linked with EDMA crosslinking agent in chloroform and added AIBN as an initiator. The mixture was refluxed until the temperature reached 110oC. Furthermore, the resin was acidified to realease Fe (III) for 24 hours using HCl and checked everyday with UV-Vis spectrophotometry. In the present test, the filtrate from acidification process was added KSCN as a complexing agent, the colour of filtrate turned into red indicating the presence of Fe metal released from the resin. The reaction is as follow.11
3SCN– + Fe3+ → Fe(SCN)3
The test was performed until no longer red complex was formed, the colour of filtrate was clear when the addition of KSCN was undertaken, it means that there was no Fe metal released. Acid compound of polyeugenoxy acetate is able to bind more Fe (III) ion templates than other functonal polymers, this was indicated by the high absorbance value in template release process using acid. The resin which has been discharged the template was then dried and can be used as an adsorbent to adsorb metals.
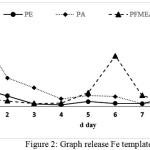 |
Figure 2: Graph release Fe template Click here to View figure |
Materials Characterisation
Analysis of FTIR
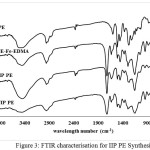 |
Figure 3: FTIR characterisation for IIP PE Synthesis Click here to View figure |
In Figure 3 it appears that the -OH polieugenol spectrum decreases in intensity when attached to Fe-EDMA, this intensity then rises again after the PE-Fe-EDMA polymer is released Fe-containing iron ions to produce IIP. The IIP spectrum is sharper than NIP, it shows the role of -OH in IIP work. It also shows that there is a difference in Polyugenol emerging acid carbonyl spectrum after crosslinked with EDMA, this is because EDMA has an acidic carbonyl group that produces peak at wave number around 1700 cm-1.
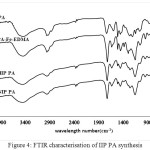 |
Figure 4: FTIR characterisation of IIP PA synthesis Click here to View figure |
In Figure 4, there is a difference in the carbonyl acid spectra of 1720 cm-1 on polieugenoxy acetate acid which becomes sharper after crosslink with EDMA. This is because EDMA also has an acid carbonyl group. In Figure 5 the PFMEA spectrum difference appears after crosslinks of carbonyl ester to shift close to the acid spectrum (1700 cm-1). This shift occurs because of the bonds / carbonyl interactions with the atoms of the EDMA crosslinker.
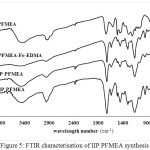 |
Figure 5: FTIR characterisation of IIP PFMEA synthesis Click here to View figure |
Analysis of SEM
One of functional polymers analysed using SEM was PA presented in figure 6. Before the polymer PA changed to become IIP it has ununiform pores, but after the PA polymer became IIP the pores size was shown clearly become more uniform, regular and spread out. This could be because of the role of Fe bound and then released from the adsorbent, this result is in accordance with the research conducted by Djunaidi, et al (2016) 8.
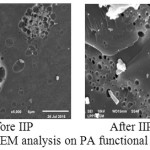 |
Figure 6: SEM analysis on PA functional polymer Click here to View figure |
Analysis of Surface Area Using BET Method
One of functional polymers analysed its surface area was PA performed using BET method. The result is as follows: IIP PA = 39.577 m²/g, NIP PA = 2.652 m²/g, and NIP PA acid (immersion with HCl) = 5.353 m²/g. It can be seen that the surface area of IIP PA decreased compared to NIP PA and NIP PA acid. Based on this result, it shows that IIP PA synthesis result underwent reorganization compared to NIP PA.
Selectivity of Metallic ion Adsorption
The selectivity of Fe metal ion adsorption based on the variation of functional polymer used can be seen in Table 1. It is shown in Table 1 that the functional polymer of polieugenoxy acetic acid (PA) produced the greatest selectivity compared to other functional polymers. This could be since the acetic group has a chemical affinity for Fe (III) ions10. The selectivity adsorption was calculated with formula %adsorption Fe ion was divided by %adsorption of competitor metal ion.
Table 1: The selectivity of Fe metal ion adsorption based on functional polymers
| Selektivity | IIP PE | NIP PE | IIP PA | NIP PA | IIP PFMEA | NIP PFMEA |
| Fe-Cr | 4,03 | 6,10 | ~ | 14,95 | 11,90 | 5,30 |
| Fe-Cd | 11,51 | 2,02 | 82,92 | 19,00 | 11,25 | 17,31 |
| Fe-Pb | 1,12 | 1,38 | 4,43 | 2,19 | 1,96 | 1,57 |
It is seen that the PE IIP sequence of similarity is similar to that of the study using the PEGDE croslinker8. This is due to the large number of OH groups in the polymer causing less affinity for Cd and its greater affinity for Pb8. Based on theory of HSAB, Fe(III) and Cr(III) include in the group of hard acids which generally have a small atomic radius called hidrated atomic radius Fe(III) 0,66 Å and Cr(III) 0,62 Å. In the meantime, Cd(II) belongs to the soft acid group and Pb(II) enters in the medium acid group wich generally has a larger atomic radius, i.e, the hydrated atomic radius of Cd (II) and Pb(II) are respectively 0,96 Å and 1,20 Å 12. The results showed that the adsorption selectivity of Fe on Fe-Cr was higher than that of on Fe-Cd and Fe-Pb, with the a selectivity sequence Fe-Cr > Fe-Cd > Fe-Pb. The highest result of adsorption selectivity of Fe on Fe-Cr indicated that the imprints formed were appropriate with the template, that is the adsorbent was suitable only for Fe metal. Although the Fe and Cr metals are in the same group with the similar properties but the imprints of the adsorbents are highly selective for Fe metals. The adsorption selectivity of Fe metal on Fe-Cd binary metal mixture was greater than that of on Fe-Pb, this is because Fe is categorized as a hard acid group with the hydrated atomic radius smaller than Cd categorized as soft acid groups and Pb in a medium acid group/borderline which has the hydrated atomic radius larger 12. Cd metal has smaller hydrated atomic radius than that of Pb metal, this is the reason that the adsorption of Fe is greater on Cd than Pb. The larger the hydrated atomic radius of the competitor metals it will prevent the Fe metal to enter the template, which further will cause the Fe metal selectivity becomes low.
Conclusion
The adsorption selectivity of Fe metal ion on plyeugenoxy acetic acid (PA) with the EDMA crosslinker was greater than that of the poly-thiphene 2-methyl-2-eugenoxy acetate (PFMEA) and polyeugenol (PE). The adsorption selectivity of Fe was greater on Fe-Cr than on Fe-Cd and Fe-Pb.
Acknowledgments
We would like to express sincere gratitude to Ministry of Riset and Technology Indonesia for Competitive Grant (Fundamental Grant 2017)
References
- Ghaee, A.; Niassar, M. S.; Barzin, J.; dan Zarghan, A. App. Surf. Sci. 2012, 258 (19), 7732–7743.
CrossRef - Fan, H.; Sun, T. Korean J. Chem. Eng, 2012, 29 (6): 798–803.
CrossRef - Harera; Rizal, L.; Sudiarti, T.; Wulandari, M. Al Kimiya. 2015, 2 (1), 30–39.
- Djunaidi, M. C.; Jumina; Siswanta, D.; Ulbricht, M. Orient. J. Chem. 2016, 32 (1), 77-84.
- Djunaidi, M.C.; Khabibi; Ulumuddin, I. IOP Conf. 2017 Ser: Mater. Sci. Eng. 172012032, 2017.
- Djunaidi, M. C.; Jumina; Siswanta, D. ; Ulbricht, M., AIP Conference Proceedings. 1699, 060001, 2016.
CrossRef - Djunaidi, M. C.; Jumina; Siswanta, D.; Ulbricht, M. Asian J. Chem. 2015, 27(12), 4553–4562.
CrossRef - Djunaidi, M. C.; Jumina; Siswanta, D.; Ulbricht, M. Indones.J.Chem, 2015, 15(3), 305–314.
CrossRef - Djunaidi, M. C.; Siswanta, D.; Jumina. Orient. J. Chem. 2015, 31(4), 2223–2229.
CrossRef - Djunaidi, M.C.; Lusiana, R.A.; Wibawa, P.J., Siswanta, D.; Jumina. Reaktor. 2010, 13(1), p. 16-23
CrossRef - Svehla, G. Vogels Text-Book Of Macro And Semimicro Qualitative Inorganic Analysis-5th Edition, 1979. Longman, London and New York.
- Persson, Ingmar. Pure Appl. Chem., 2010. 82 (10): 1901–1917.
CrossRef

This work is licensed under a Creative Commons Attribution 4.0 International License.









