Synthesis, Characterization and Antibacterial Evaluation of some Novel Benzimidazole Derivatives Containing 1,3,4-Thiadiazole Moiety.
Muayed Ahmed Redayan1 , Wassan Baqir Ali2 and Ahmed Mudhafar Mohammed2
, Wassan Baqir Ali2 and Ahmed Mudhafar Mohammed2
1Department of Chemistry, College of education for pure sciences, Diyala University, Iraq.
2Department of Chemistry, College of Sciences, Diyala University, Iraq.
Corresponding Author E-mail: mredayan@gmail.com
DOI : http://dx.doi.org/10.13005/ojc/330656
A series of novel 5-amino-1,3,4-thiadiazole-2-thiol and 1,3,4-thiadiazole-2,5-dithiol derivatives of benzimidazole were synthesized through nucleophilic substitution reaction of 5-substituted-2-(chloromethyl)-1H-benzimidazole, structures of the synthesized compounds were proved by spectral methods of analysis ( FT-IR, 1H and 13C NMR ). All the target compounds were screened for their antibacterial activity toward gram-negative (E.coli, P. aeruginosa) and gram-positive (B. subtilis, S. aureus) bacteria, most of the synthesized derivatives exhibited good to moderate activity toward both gram-positive (B. subtilis, S. aureus) and gram-negative (E.coli, P. aeruginosa) bacteria.
KEYWORDS:Benzimidazole; Bis Benzimidazole; 1,3,4-Thiadiazole; Antibacterial Activity
Download this article as:| Copy the following to cite this article: Redayan M. A, Ali W. B, Mohammed A. M. Synthesis, Characterization and Antibacterial Evaluation of some Novel Benzimidazole Derivatives Containing 1,3,4-Thiadiazole Moiety. Orient J Chem 2017;33(6). |
| Copy the following to cite this URL: Redayan M. A, Ali W. B, Mohammed A. M. Synthesis, Characterization and Antibacterial Evaluation of some Novel Benzimidazole Derivatives Containing 1,3,4-Thiadiazole Moiety. Orient J Chem 2017;33(6). Available from: http://www.orientjchem.org/?p=40112 |
Introduction
The benzimidazole nucleus consider one of the most significant and important N-containing fused organic compounds in a large number of synthetic pharmaceutical materials and natural products. [1, 2] Benzimidazole ring count a significant heterocyclic pharmacophore in the drug discovery, these compounds which carrying diverse substituents in the structure of benzimidazole are associated with a wide range of biological activities including: antifungal, [3, 4, 5] anticancer, [6, 7, 8] anti-inflammatory, [9, 10] antioxidant, [11, 12, 13] anti-bacterial, [14, 15, 16, 17] anti-viral, [18, 19] anticoagulant, [20] and anti-hypertensive properties . [21]
Despite of many attempts to develop and discover new structural type in the search for great and more effective antimicrobials, benzimidazoles still consider as one of the most versatile and significant type of compounds against microbes. [22, 23]
The benzimidazole containing compounds as a structural motif have been widely used in medicinal chemistry and drugs development. Amongst the benzimidazole derivatives 2-chloromethyl benzimidazole show a considerable importance in biological chemistry, they are important intermediates in the synthesis of many biologically active compounds. [24]
Designing new compounds in order to deal with resistant bacteria has become one of the most significant and great areas of antibacterial research today. Because the resistance of pathogenic bacteria toward common and available antimicrobial drugs is quickly becoming a major worldwide problem, so the discovery of new and potent antibacterial agent is more challenging and demanding for pharmacists and chemists nowadays. [25]
Materials and Methods
Melting points were determined using stuart smp3 apparatus and are uncorrected, FT-IR spectra were recorded on shimadzu FT-IR spectrophotometer, 1H and 13C –NMR spectra were recorded on brucker 300 MHz spectrophotometer using (DMSO) as a solvent and TMS as internal reference. The compounds were checked for their purity on silica gel TLC plates and the visualization of spots performed by using UV light.
General Method for the Synthesis of 5-Substituted-2-(Chloromethyl)-1H-Benzimidazole 1(a-d)[15, 26, 27]
A mixture of 4-(un)substituted-o-phenylenediamine (0.05 mole) and chloroacetic acid (0.05 mole) was dissolved in ( 25 ml) 4N HCl and refluxed for 4hrs. The completion of the reaction was checked by using T.L.C ( mobile phase:ethyl acetate: hexane 2:1). The reaction mixture was allowed to cool down and neutralized with ammonium hydroxide solution, the precipitate appeared was dried and recrystallized from (methanol/water).
2-(chloromethyl)-1H-benzimidazole (1a)
yellow crystals, m.p: 147-150°C, IR (KBr, cm-1): N-Hstr (3133), aromtic C-Hstr ( 3050), aliphatic C-Hstr (2900, 2846), C=Nstr (1625), aromatic C=Cstr (1520, 1446), C-Clstr (640), Yield: 93%.
2-(chloromethyl)-5-methyl-1H-benzimidazole (1b)
Dark brown crystals, m.p:130-134°C, IR (KBr, cm-1): N-Hstr (3147), aromtic C-Hstr (3045), aliphatic C-Hstr(2919, 2850), C=Nstr (1622), aromatic C=Cstr (1522, 1447), C-Clstr(646), yield: 90%.
2-(chloromethyl)-1H– benzimidazole-5-carboxylic acid (1c)
Brown crystals, m.p: 290-293°C, IR (KBr, cm-1): N-Hstr (3379), OHstr (2600-3350), aromtic C-Hstr ( 3040), aliphatic C-Hstr (2966, 2806), C=Ostr (1681), C=Nstr (1614), aromatic C=Cstr (1425-1573), C-Clst (675), yield: 88%.
2-(chloromethyl)-5-nitro-1H-benzimidazole (1d)
Dark yellow crystals, m.p:168-170°C, IR (KBr, cm-1): N-Hstr (3259), aromtic C-Hstr (3028), aliphatic C- Hstr (2980, 2800), C=Nstr (1624), C=Cstr (1446, 1471), NO2 str (1334, 1508), C-Clstr (690), yield: 85%.
Synthesis of 5-amino-1,3,4-thiadiazole-2-thiol (2)[28]
A mixture of (4 g, 0.04 mole) of thiosemicarbazide and (4.66g, 0.04 mole) of anhydrous Sodium carbonate were dissolved in 50 ml of absolute ethanol. (6.4 g, 0.08 mole) of carbon disulfide was then added to this solution. The resulting mixture was then refluxed for 11 hrs, subsequently the reaction mixture was allowed to cool down at R.T. Most of solvent was distilled off under reduced pressure and the residue was dissolved in 40 ml of distilled water and then carefully acidified with cold conc. Hydrochloric acid to give pale yellow precipitate. The product was then filtered and washed with cold water, then recrystallized from hot water, T.L.C (mobile phase: ethyl acetate: hexane 2:1 ). M.p: 232-234°C, IR (KBr, cm-1): N-Hstr (3399, 3279), S-Hstr (2529), C=Nstr (1601), C=Sstr (1363), C-Sstr (670), yield 83%.
General method for the Synthesis of compounds 3(a-d)[29]
5-amino-1,3,4-thiadiazole-2-thiol (2) (5 mmole) and fused sodium acetate (5 mmole) were added To the solution of compound (3) (5 mmole) in absolute ethanol (35 ml), the mixture was refluxed for 12 hrs, the reaction completion was checked by using T.L.C (mobile phase: ethyl acetate: hexane 2:1), then the reaction mixture was cooled and the precipitate was collected by filtration and dried.
5-[(1H-benzimidazol-2-ylmethyl)sulfanyl]-1,3,4-thiadiazol-2-amine (3a)
Yellow-brown crystals, m.p: 196-198°C, IR (KBr, cm-1): NH2 str (3252), aromtic C-Hstr (3085), aliphatic C-Hstr (2954, 2918), C=Nstr (1622), aromatic C=Cstr (1526, 1434), C-Sstr (671), yield 53%.
5-{[(5-methyl-1H-benzimidazol-2-yl)methyl]sulfanyl}-1,3,4-thiadiazol-2-amine (3b)
Brown crystals, m.p: >350°C, IR (KBr, cm-1): NH2 str (3342, 3264), N-Hstr (3133), aromtic C-Hstr (3080), aliphatic C- Hstr (2972, 2918), C=Nstr (1610), aromatic C=Cstr (1556, 1449), C-Sstr (621), yield 47%.
2-{[(5-amino-1,3,4-thiadiazol-2-yl)sulfanyl]methyl}-1H-benzimidazole-5-carboxylic acid (3c)
Brown crystals, m.p: 240 dec.°C, IR (KBr, cm-1): OHstr (2800-3550), NH2 str (3442, 3265), N-Hstr (3147), aromtic C-Hstr (3051), aliphatic C-Hstr (2941, 2864), C=Ostr (1697), C=Nstr (1616), aromatic C=Cstr (1571, 1512, 1421), C-Sstr (671). 1HNMR (DMSO-d6) δ ppm: 3.9 ( s, 2H, CH2), 4.4 ( s, 1H, N-H), 4.58 (s, 2H, NH2), 7.6 – 8.1 (m, 3H, Ar-H), 12.9 (s, 1H, OH), yield 45%.
5-{[(5-nitro-1H-benzimidazol-2-yl)methyl]sulfanyl}-1,3,4-thiadiazol-2-amine (3d)
Light brown crystals, m.p: 214°C, IR (KBr, cm-1): NH2 str (3406, 3284), N-Hstr (3107), aromtic C-Hstr (3057), aliphatic C-Hstr (2968, 2800), C=Nstr (1627), aromatic C=Cstr (1537, 1469), N02str (1338, 1514), C-Sstr (640), 1HNMR (DMSO-d6) δ ppm: 3.37 (s, 1H, N-H), 4.3 (s, 2H, CH2), 7 ( s, 2H, NH2), 7.6-8.1 (m, 3H, Ar-H), yield 90%.
Synthesis of 1,3,4-thiadiazole-2,5-dithiol (4)[30]
A mixture of carbon disulfide (0.04 mole, 30 ml) and hydrazine hydrate 80% (0.04 mole,10 ml) with pyridine (100 ml) was heated under reflux for 5 hours. Then excess solvent was distilled off and the solid obtained was separated by adding (50 ml ) of water and (10 ml) of hydrochloric acid. The precipitate was then filtered, dried and recrystallized from ethanol. T.L.C (mobile phase: ethyl acetate: hexane 2:1). Yellow crystals, m.p:163-166°C, IR (KBr, cm-1): N-Hstr (3055), S-Hstr (2732), C=Nstr (1622), C=Sstr (1266), C-Sstr (677), yield 85%.
General method for the Synthesis of compounds 5(a-d) [29]
To the solution of compounds (1) (0.02mole ) in absolute ethanol(60 ml) the 1,3,4-thiadiazole-2,5-dithiol (4) (0.01 mole) and fused sodium acetate (0.02 mole) were added and the mixture was refluxed for 12 hrs, after completion of the reaction which was checked by T.L.C (mobile phase: ethyl acetate: hexane 2:1 ) the mixture was allowed to cool down and cooled in ice bath, the product was collected by filtration and dried.
2,2′-[1,3,4-thiadiazole-2,5-diylbis(sulfanediylmethanediyl)]bis(1H-benzimidazole) (5a)
Yellow-brown crystals, m.p: 218-220 dec.°C, IR (KBr, cm-1): N- Hstr (3420), aromtic C-Hstr (3100), aliphatic C-Hstr (2978, 2930), C=Nstr (1622), aromatic C=Cstr (1526, 1437), C-Sstr (746), 1HNMR (DMSO-d6) δ ppm: 3.4 (s, 2H, CH2), 4.8 (s, 1H, N-H), 7.1-7.7 (m, 8H, Ar-H) yield 47%.
2,2′-[1,3,4-thiadiazole-2,5-diylbis(sulfanediylmethanediyl)]bis(5-methyl-1H-benzimidazole)(5b)
Light brown crystals, m.p: >300°C, IR (KBr, cm-1): N- Hstr (3417), aromtic C-Hstr (3072), aliphatic C-Hstr (2976), C=Nstr (1637), aromatic C=Cstr (1618, 1562), C-Sstr (619), yield 44%.
2,2′-[1,3,4-thiadiazole-2,5-diylbis(sulfanediylmethanediyl)]bis(1H-benzimidazole-5-carboxylic acid) (5c)
Light green. M.p: 284-285°C, IR (KBr, cm-1): N- Hstr (3431), OHstr (2500-3600), aromtic C-Hstr (3074), aliphatic C-Hstr (2974, 2889, 2804), C=Ostr (1699), C=Nstr (1631), aromatic C=Cstr (1616, 1564), C-Sstr (769), 1HNMR (DMSO-d6) δ ppm: 5.09 (s, 2H, CH2), 5.3 (s, 1H, N-H), and 7.69-8.3 (m, 6H, Ar-H). 13CNMR (DMSO-d6) δ ppm: 29.5 (CH2), 114.3, 116.2, 124.5, 126.7, 134.1, 137.1(Ar-C), 152.1(C=N of benzimidazole ), 164.3(C=N of thiadiazole , 167.2(C=O), yield 73%.
2,2′-[1,3,4-thiadiazole-2,5-diylbis(sulfanediylmethanediyl)]bis(5-nitro-1H-benzimidazole)(5d)
Light brown crystals, m.p: 165°C. IR (KBr, cm-1): N- Hstr (3419), aromtic C-Hstr (3099), aliphatic C-Hstr (2974, 2918), C=Nstr (1627), aromatic C=Cstr (1597, 1471 ), NO2str (1346, 1519), C-Sstr (738), 1HNMR (DMSO-d6) δ ppm: δ 3.6 (s, 1H, N-H), δ 5.0 (s, 2H, CH2), and δ 7.6-8.4 ( m, 6H, Ar-H ). 13CNMR (DMSO-d6) δ ppm: 31.0 (CH2), 104.7, 108, 116.1, 117.9, 118.2, 129.4 (Ar-C), 142.6 (C=N of benzimidazole), 164.5 (C=N of thiadiazole). Yield 78%.
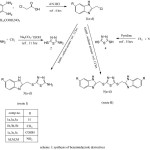 |
Scheme 1: synthesis of benzimidazzole derivatives |
Result and Discussion
The reaction sequence for various title compounds is summerized in (scheme 1). The starting material 5-substituted-2-(chloromethyl)-1H-benzimidazole 1(a-d) was synthesized according to a reported procedure through the reaction of 4-(un)substituted-o-phenylenediamine with chloroacetic acid.[15, 26, 27] Structure of compounds 1(a-d) was confirmed by comparison of its physical and spectral data with the reported ones, [26, 27, 31] nucleophilic substitution reaction of compound 1(a-d) with 5-amino-1,3,4-thiadiazole-2-thiol (2) yielded 5-{[(5-substituted-1H-benzimidazol-2-yl)methyl]sulfanyl}-1,3,4-thiadiazol-2-amine 3(a-d) ( route I), the structure of compounds 3(a-d) was confirmed by its IR, 1H-NMR, the IR spectra of the compound (3c) exhibited a broad band at (2800-3550 cm-1 for carboxylic OH ), (3442, 3265cm-1 for NH2), ( 3147 cm-1 for benzimidazole N-H), (1697 cm-1 for C=O) and band at (671 cm-1 for C-S ). 1HNMR spectra showed the following chemical shifts at: δ 3.9 (s, 2H, CH2), δ 4.4 (s, 1H, N-H ), δ 4.58 (s, 2H, NH2), δ 7.6 – 8.1(m, 3H, Ar-H), and weak signal at δ 12.9 for carboxylic OH, and the IR spectra of the compound (3d) exhibited the following bands: (3406, 3284 cm-1) for NH2, (3107 cm-1) for benzimidazole N-H, and two peaks at (1338, 1323 cm-1) corresponding for NO2 group. Whereas the 1HNMR spectra for this compound revealed the following peaks: δ 3.37 (s, 1H, N-H), δ 4.3 (s, 2H, CH2), δ 7 ( s, 2H, NH2), and multiplet signals at δ (7.6-8.1) corresponding for aromatic protons.
The ( route II ) also included nucleophilic substitution reaction of compound 1(a-d) with 1,3,4-thiadiazole-2,5-dithiol (4) yielded 2,2′-[1,3,4-thiadiazole-2,5-diylbis(sulfanediylmethanediyl)]bis(5-subsituted-1H-benzimidazole) 5(a-d), The structure of compounds 5(a-d) was confirmed by its IR, 1H-NMR and 13C-NMR, The IR spectra of the compound (5c) showed a broad band at 2500-3600 cm-1 for carboxylic OH group, 3431 cm-1 for benzimidazole N-H, 1699 cm-1 for C=O group, 1631 cm-1 for C=N group, and band at 769 cm-1 for C-S group. Whereas the 1HNMR spectra exhibited the following chemical shifts: δ 5.09 (s, 2H, CH2), δ 5.3 (s, 1H, N-H), and δ 7.69-8.3 (m, 6H, Ar-H). The signals in 13CNMR was appeared at around: δ 29.5 accounted for the methylene group (-CH2-), signals at (114.3, 116.2, 124.5, 126.7, 134.1, 137.1) could be for benzene ring, also 13CNMR spectra showed signal about 152.1 and 164.3 for (-C=N) of benzimidazole and thiadiazole ring respectively, whereas signal at 167.2 accounted for (-C=O) group.
The IR spectra for the compound (5d) found have the following bands: 3419 cm-1 for N-H group , 1627 cm-1 for C=N group, and two bands observed at (1346, 1315 cm-1 ) accounted for NO2 group. The 1HNMR spectra showed the chemical shifts: δ 3.6 (s, 1H, N-H), δ 5.0 (s, 2H, CH2), and peaks at δ 7.6-8.4 ( m, 6H, Ar-H ). 13CNMR spectra result for this compound found in full agreement with its assigned structures. Beginning with signal appeared at about δ 31.0 corresponding for the methylene group (-CH2-), signals of benzene ring appeared at about δ (104.7, 108, 116.1, 117.9, 118.2, 129.4) whereas the chemical shifts at about 142.6 and 164.5 related to benzimidazole and thiadiazole (-C=N ) group respectively. The purity of the synthesized compounds was monitored by TLC. Physical properties of the synthesized compounds are shown in Table 1.
Table 1: Physical properties of the compounds
|
Comp no. |
R |
m.p (oC) |
M.wt(g/mole) |
Mol.Formula |
Color |
Yield % |
|
1a |
H |
147-150 |
166.61 |
C8H7ClN2 |
yellow |
93% |
|
1b |
CH3 |
130-134 |
180.64 |
C9H9ClN2 |
dark brown |
90% |
|
1c |
COOH |
290-293 |
210.62 |
C9H7ClN2O2 |
brown |
88% |
|
1d |
NO2 |
168-170 |
211.61 |
C8H6ClN3O2 |
dark yellow |
85% |
|
2 |
232-234 |
133.19 |
C2H3N3S2 |
pale yellow |
83% |
|
|
3a |
H |
196-198 |
263.34 |
C10H9N5S2 |
yellow-brown |
53% |
|
3b |
CH3 |
>350 |
277.36 |
C11H11N5S2 |
brown |
47% |
|
3c |
COOH |
240 dec. |
307.35 |
C11H9N5S2 |
brown |
45% |
|
3d |
NO2 |
214 |
308.33 |
C10H8N6O2S2 |
light brown |
90% |
|
4 |
163-166 |
150.23 |
C2H2N2S3 |
yellow |
85% |
|
|
5a |
H |
218-220 |
410.53 |
C18H14N6S3 |
yellow-brown |
47% |
|
5b |
CH3 |
>300 |
438.59 |
C20H18N6S3 |
light brown |
44% |
|
5c |
COOH |
284-285 |
498.55 |
C20H14N6O4S3 |
light green |
73% |
|
5d |
NO2 |
165 |
500.53 |
C18H12N8O4S3 |
light brown |
78 % |
dec. : decomposed
Antibacterial Evaluation
Some of the newly synthesized compounds were tested for their in-vitro antibacterial activities against gram-negative including (Pseudomonas aeruginosa, Esherichia coli) and gram-positive including (Staphylococcus aureus, bacillus subtilis) bacteria by disc diffusion method, the concentration of the compounds used were (10 mg/ml and 100 mg/ml ). Inhibition zones were measured in millimeters and compared with (Ampicillin and ciprofloxacin) as a standard antibiotics references. The results are illustrated in ( Table 2) which demonstrates that most of compounds tested for their antibacterial activity exhibited good to moderate activities. Amongst all compounds tested (3a, 3c, 3d, 5c) showed good to moderate activity against all types of bacteria used.
Table 2: antibacterial activity of the synthesized compounds
| Comp no. | Concentration(mg / ml) |
Zone of inhibition ( in mm) |
|||
|
Gram-positive |
Gram-negative |
||||
|
S. aureus |
B. subtilis |
P. aeruginosa |
E. coli |
||
|
3a |
10 |
12 |
18 |
12 |
24 |
| 100 |
14 |
17 |
– |
25 |
|
|
3b |
10 |
– |
– |
– |
– |
| 100 |
11 |
12 |
– |
– |
|
|
3c |
10 |
– |
– |
12 |
12 |
| 100 |
22 |
23 |
18 |
19 |
|
|
3d |
10 |
12 |
19 |
11 |
22 |
| 100 |
12 |
18 |
– |
20 |
|
|
5a |
10 |
– |
15 |
– |
12 |
| 100 |
– |
– |
– |
– |
|
|
5b |
10 |
– |
14 |
– |
– |
| 100 |
12 |
15 |
– |
– |
|
|
5c |
10 |
11 |
13 |
12 |
14 |
| 100 |
30 |
30 |
15 |
22 |
|
|
5d |
10 |
– |
11 |
– |
15 |
| 100 |
– |
– |
– |
– |
|
| Ampicillin |
22 |
23 |
– |
10 |
|
| ciprofloxacin |
19 |
23 |
29 |
– |
|
| DMSO solvent |
0 |
0 |
0 |
0 |
|
Conclusion
Bnzimidazole derivatives containing 5-amino-1,3,4-thiadiazole-2-thiol and 1,3,4-thiadiazole-2,5-dithiol were synthesized by nucleophilic substitution reaction of 5-substituted-2-(chloromethyl)-1H-benzimidazole. The pharmacological study was performed to determine the effects of substituent on the antibacterial activity, most of the derivatives showed good to moderate activity toward gram-negative (E.coli, P. aeruginosa) and gram-positive (B. subtilis, S. aureus) bacteria.
Acknowledgment
Authors are thankful to Chemistry department, college of education for pure sciences, Diyala university and also Chemistry department, Sciences college, Diyala university for their help and support.
References
- Bansal Y.; Silakari O., Bioorganic & medicinal chemistry 2012, 20,6208-6236.
CrossRef - Saral H.; Özdamar Ö.; Uçar İ., Journal of Molecular Structure 2017, 1130,46-54.
CrossRef - Youssif B. G.; Abdel-Moty S. G.; Sayed I. M., Journal of Current Chemical and Pharmaceutical Sciences 2014, 4,54-64.
- Pawar N.; Dalal D.; Shimpi S.; Mahulikar P., European journal of pharmaceutical sciences 2004, 21,115-118.
CrossRef - Ansari K.; Lal C., European journal of medicinal chemistry 2009, 44,4028-4033.
CrossRef - El-Nassan H. B., European journal of medicinal chemistry 2012, 53,22-27.
CrossRef - Gellis A.; Kovacic H.; Boufatah N.; Vanelle P., European Journal of Medicinal Chemistry 2008, 43,1858-1864.
CrossRef - Rashid M.; Husain A.; Mishra R., European journal of medicinal chemistry 2012, 54,855-866.
CrossRef - Achar K. C.; Hosamani K. M.; Seetharamareddy H. R., European journal of medicinal chemistry 2010, 45,2048-2054.
CrossRef - El-Nezhawy A. O.; Biuomy A. R.; Hassan F. S.; Ismaiel A. K.; Omar H. A., Bioorganic & medicinal chemistry 2013, 21,1661-1670.
CrossRef - Ates‐Alagoz Z.; Can‐Eke B.; Coban T.; Iscan M.; Buyukbingol E., Archiv der Pharmazie 2004, 337,188-192.
CrossRef - Ayhan-KIlcigİl G.; Kuş C.; Çoban T.; Can-Eke B.; Özbey S.; Iscan M., Journal of enzyme inhibition and medicinal chemistry 2005, 20,503-514.
CrossRef - Kálai T.; Balog M.; Szabó A.; Gulyás G.; Jekő J. z.; Sümegi B. z.; Hideg K., Journal of medicinal chemistry 2009, 52,1619-1629.
- Nguyen P.; Baldeck J.; Olsson J.; Marquis R., Molecular Oral Microbiology 2005, 20,93-100.
- Gowda J.; Khader A.; Kalluraya B.; Hidayathulla S., Indian journal of chemistry 2011, 50,1491-1495.
- Kumar B. V. S.; Vaidya S. D.; Kumar R. V.; Bhirud S. B.; Mane R. B., European journal of medicinal chemistry 2006, 41,599-604.
CrossRef - Özkay Y.; Tunalı Y.; Karaca H.; Işıkdağ İ., European journal of medicinal chemistry 2010, 45,3293-3298.
CrossRef - Li Y.-F.; Wang G.-F.; He P.-L.; Huang W.-G.; Zhu F.-H.; Gao H.-Y.; Tang W.; Luo Y.; Feng C.-L.; Shi L.-P., Journal of medicinal chemistry 2006, 49,4790-4794.
CrossRef - Vitale G.; Corona P.; Loriga M.; Carta A.; Paglietti G.; Giliberti G.; Sanna G.; Farci P.; Marongiu M. E.; La Colla P., European journal of medicinal chemistry 2012, 53,83-97.
CrossRef - Mederski W. W.; Dorsch D.; Anzali S.; Gleitz J.; Cezanne B.; Tsaklakidis C., Bioorganic & medicinal chemistry letters 2004, 14,3763-3769.
CrossRef - Kumar J. R.; Jawahar L.; Pathak D., Journal of Chemistry 2006, 3,278-285.
- Ansari K.; Lal C., European journal of medicinal chemistry 2009, 44,2294-2299.
CrossRef - Göker H.; Tunçbilek M.; Süzen S.; Kus C.; Altanlar N., Archiv der Pharmazie 2001, 334,148-152.
CrossRef - El-Gohary N.; Shaaban M., European Journal of Medicinal Chemistry 2017, 131,255-262.
CrossRef - Padalkar V. S.; Borse B. N.; Gupta V. D.; Phatangare K. R.; Patil V. S.; Umape P. G.; Sekar N., Arabian Journal of Chemistry 2016, 9,S1125-S1130.
CrossRef - Mariappan G.; Hazarika R.; Alam F.; Karki R.; Patangia U.; Nath S., Arabian Journal of Chemistry 2015, 8,715-719.
CrossRef - Galal S. A.; Hegab K. H.; Hashem A. M.; Youssef N. S., European journal of medicinal chemistry 2010, 45,5685-5691.
CrossRef - Yusuf M.; Khan R. A.; Ahmed B., Bioorganic & medicinal chemistry 2008, 16,8029-8034.
CrossRef - Eisa H. M.; Barghash A.-e. M.; Badr S. M.; Farahat A. A., Indian journal of chemistry 2010, 49,1515-1525.
- Salimon J.; Salih N.; Hameed A.; Ibraheem H.; Yousif E., Journal of applied sciences research 2010, 6,866-870.
- PETKAR K.; PAREKH P.; KUMARI P. M. A.; BARO A., Int. J. Pharm. Pharm. Sci. 2013, 5,115-119.

This work is licensed under a Creative Commons Attribution 4.0 International License.









