Synthesis and Anti-Inflammatory Activity of Hydrazide-Hydrazones Bearing Anacardic Acid and 1,2,3-Triazole Ring Based Hybrids
A. L. V. Kumar Reddy1 and Niren E. Kathale2
and Niren E. Kathale2
1Department of Chemistry, Gondwana University, Gadchiroli-4420 605, Maharashtra, India. 2Department of Chemistry, Sardar Patel Mahavidyalaya, Chandrapur, Maharashtra, India. Corresponding Author E-mail: alvkumar2016@gmail.com
DOI : http://dx.doi.org/10.13005/ojc/330628
A novel series of hydrazide-hydrazone derivatives 39-50, linked with anacardic acid motif and 1,2,3-triazole ring were synthesized by reacting 4-(1-(2-methoxy-6-pentadecylbenzyl)-1H-1, 2, 3-triazol-4-yl)benzaldehyde with 2-phenyl aceto hydrazides and benzohydrazides. The structures of the newly synthesized hydrazide-hydrazone derivatives 39-50 were confirmed by 1H NMR, MS and IR spectroscopic tools. These compounds were also evaluated for their anti-inflammatory activity by carrageenan paw edema method.
KEYWORDS:Anacardic Acid; Anti-Inflammatory Activity; Hydrazide-Hydrazone; 1,2,3-Triazole
Download this article as:| Copy the following to cite this article: Reddy A. L. V. K, Kathale N. E. Synthesis and Anti-Inflammatory Activity of Hydrazide-Hydrazones Bearing Anacardic Acid and 1,2,3-Triazole Ring Based Hybrids. Orient J Chem 2017;33(6). |
| Copy the following to cite this URL: Reddy A. L. V. K, Kathale N. E. Synthesis and Anti-Inflammatory Activity of Hydrazide-Hydrazones Bearing Anacardic Acid and 1,2,3-Triazole Ring Based Hybrids. Orient J Chem 2017;33(6). Available from: http://www.orientjchem.org/?p=40276 |
Introduction
Hydrazones are a class of organic compounds that contain the azomethine (–NHN=CH-) proton and they are formed by the action of the appropriate substituted hydrazines/hydrazides on aldehydes or ketones by heating in solvents like ethanol, methanol, tetrahydrofuran, butanol, glacial acetic acid, ethanol-glacial acetic acid1. Hydrazone and acylhydrazone derivatives have been the subject of considerable interest in the development of novel compounds with anti-inflammatory2-6, antidepressant7, analgesic8, anticonvulsant9, antimicrobial10, antifungal11, antitumor12, antioxidant13, antimalarial 14, antiplatelet, and antiviral, antimycobacterial and vasodilator activities 15.
Triazoles and their derivatives are well known to be effectual pesticides, fungicides and insecticides16-18. They have been reported to be antagonists and agonists of receptors19-21, inhibitors of enzymes 22, and were found to exhibit antihistaminic, cytotoxic, neuroleptic, antiproliferative and anti-HIV-1 activities 23-27as well as their antimicrobial, antitumor, antibacterial, anti-inflammatory, antiviral and antifungal agents 28-34.
Anacardic acid, chemically named as 6-pentadecyl salicylic acid, is a natural product found in cashew nut shells. Anacardic acid has multiple roles including histone acetyltransferase, the inhibition of lipid synthesis, prostaglandin endoperoxide synthase and the activation of aurora kinase A 35–40. This compound is often associated with antibacterial, anticancer effect, anti -inflammatory, anti-tumor, molluscicidal, and anti-microbial activities41-44.
Prompted by the various biological activities associated with hydrazones, 1,2,3-triazole and anacardic acid derivatives, we report herein the synthesis, characterization and anti-inflammatory activity of hydrazide-hydrazone derivatives embedded with pharmacological active scaffolds viz., anacardic acid and 1,2,3-triazole ring. The structural determination of the synthesized, hydrazide-hydrazone compounds have been confirmed by 1H NMR, mass and IR spectroscopic tools.
Results and Discussion
Chemistry
The synthesis of hydrazide-hydrazone derivatives 39-50 linked with anacardic acid motif and 1,2,3-triazole ring is presented in scheme-1. The preparation of (i) 2-phenyl-acetohydrides 11-15 (ii) benzohydrazides 30-36 (iii) the key intermediate, 4-(1-(2-methoxy-6-pentadecylbenzyl)-1H-1, 2, 3-triazol-4-yl)benzaldehyde 38 was prepared following the literature procedure reported by us recently46, 47. Coupling of triazole-aldehyde 38 with 2-phenyl aceto hydrazides 11-15 and benzohydrazides 30-36 was carried out by grinding in mortar and pestle (solvent free conditions) in presence of minimum quantity of ethanol for 2-3 min resulted in the formation of hydrazide-hydrazone derivatives 39-50 in quantitative yields. The structural determination of synthesized derivatives 39-50 was determined by 1H NMR, mass and IR spectral data. As an example, among benzohydrazide series 44-50, the 1H NMR assignment of (E)-N’-(4-(1-(2-methoxy-6-pentadecylbenzyl)-1H-1,2,3-triazol-4-yl)benzylidene)-3,4,5-trimethoxybenzohydrazide 48 is described here, the singlet signals with one proton integration resonating at 11.70 ppm, 8.45 ppm and 8.36 ppm is assigned to the following groups viz.,–CONH-, -N=CH– and triazole ring respectively. In the aromatic region, the protons resonating at 7.93 ppm and 7.75 ppm as doublets with two proton integration is assigned to the para-substituted phenyl ring flanked between hydrazone group and the triazole ring, while the protons resonating at 7.22 ppm (singlet, 2H), 7.28 ppm (triplet, 1H), 6.93 ppm (doublet, 1H) and 6.84 ppm (doublet, 1H) is assigned to 3,4,5-trimethoxy phenyl ring and anacardic acid ring protons. In the aliphatic region, the proton signals at 0.80 ppm (triplet, 3H), 1.33-1.12 ppm (multiplet, 26H), and 2.71 ppm (triplet, 2H) is assigned to the side chain of the anacardic ring. The proton signals at 3.71 (singlet, 3H), 3.83 (singlet, 3H), 3.84 (singlet, 3H) and 5.58 (singlet, 2H) corresponds to the methoxy groups and methylene group (-CH2). The IR spectra of compound 48 showed characteristic peaks at 3228,1648, 1602, 1335 and 1088 cm-1 corresponds to –CONH, -C=O, -C=N, -C-N and –C-O-C groups respectively. The mass spectral data of compound 48 showed m/z, 712.4 (M+H)+ is in agreement with the desired molecular formulae.
In case of 2-substituted phenyl- acetohydrazide-hydrazone compounds viz., 39-43, the 1H NMR showed two sets of signals indicating the possibility of equilibrium and interconversion between rotamers (and/or configurational isomers) in solution. These compounds were found to exist as a mixture of two rotameric forms in solution 48, 49 e.g. antiperiplanar (ap) and synperiplanar (sp). As an example, the 1H NMR spectra of (E)-N’-(4-(1-(2-methoxy-6-pentadecylbenzyl)-1H-1,2,3-triazol-4-yl)benzylidene)-2-(4-nitrophenyl)acetohydrazide (42) is described here, the proton signals resonating at 11.69 (* 11.53, singlet, 1H) ppm, 8.36 (* 8.22, singlet, 1H) ppm, 8.13 (* 7.85, singlet, 1H) ppm and 4.18 (* 3.74, singlet, 2H) corresponds to -CO-NH– and –N=CH– , -CH2 group of –para-nitro phenyl groups respectively. The anacardic acid phenyl ring protons appeared at 7.30 ppm (triplet, 1H), 6.94 ppm (doublet, 1H) and 6.86 ppm (doublet, 1H) while the protons resonating at 8.20 ppm, 7.92 ppm, 7.72 ppm and 7.60 ppm as doublets with two proton integration is assigned to the para-nitro phenyl ring and to the para substituted phenyl ring flanked between the triazole ring and the hydrazone group.
The aliphatic side chain of the anacardic acid moiety appeared in the expected aliphatic region, the singlet proton signals in the region 5.60 ppm and 3.85 ppm corresponds to the methylene group (linking anacardic acid and triazole ring) and methoxy group respectively. The mass spectra of these compounds showed (M+1) peaks and are in agreement with their molecular formula. The IR spectral data also were found to be in the expected range and gave the evidence for the expected functional groups.
Determination of E– or Z- geometry of the C=N bond in hydrazide-hydrazone derivatives 39-40, by 1H NMR was based on the earlier report, that N-acylhydrazones derived from aromatic aldehydes in solution remained in the E form, because of the hindered rotation on the imine bond 50, we considered E– geometry in our case48.
Anti-inflammatory Activity
The synthesized 2-phenylacetohydrazide-hydrazone 39-43 and benzohydrazide-hydrazone derivatives 44-50 were evaluated for anti-inflammatory activity (by carrageenan paw edema method, dosage: at 10 mg/Kg po) and the results are presented in Table 1. Compounds 41 and 43 bearing 2-phenylacetohydrazides with substitution R = 2-Cl-4-Fluoro and tetrahydrofuran ring exhibited excellent anti-inflammatory activity and compounds 39,40 and 42 (bearing 2-phenylacetohydrazides with substitution R = H, 3,5-CH3 and 4-NO2) displayed good anti-inflammatory activity and the remaining compounds in the series i.e compounds 44-50 bearing benzohydrazides displayed moderate to weak anti-inflammatory activity.
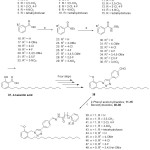 |
Scheme 1: Synthesis of novel series of hydrazide-hydrazone derivatives 39-50 |
Experimental conditions: a) conc; H2SO4, EtOH; b) NH2-NH2, EtOH; c) Hydrazides 11-15 and 30-36, grinding, minimum qty; EtOH, 2-3 min
Table 1: Results of Anti-inflammatory activity of hydrazide-hydrazone derivatives 39-50
|
Treatments |
1 hr |
2 hr |
3 hr |
|
Carrageenan Control |
0.98 ± 0.09 |
1.25 ± 0.12 |
2.55 ± 0.12 |
|
39 |
0.48 ± 0.32 |
0.60 ± 0.22 |
0.74 ± 0.41 |
|
40 |
0.52 ± 0.22 |
0.64 ± 0.22 |
0.76 ± 0.16 |
|
41 |
0.60 ± 1.45 |
0.80 ± 0.44 |
0.95 ± 0.28 |
|
42 |
0.56 ± 0.32 |
0.68 ± 0.55 |
0.74 ± 0.26 |
|
43 |
0.66 ± 0.11 |
0.84 ± 0.19 |
1.05 ± 0.24 |
|
44 |
0.28 ± 0.52 |
0.38 ± 0.36 |
0.54 ± 0.14 |
|
45 |
0.36 ± 0.20 |
0.44 ± 0.18 |
0.62 ± 0.24 |
|
46 |
0.22 ± 0.12 |
0.32 ± 0.32 |
0.52 ± 0.21 |
|
47 |
0.18 ± 0.33 |
0.30 ± 0.15 |
0.48 ± 0.40 |
|
48 |
0.41 ± 0.18 |
0.52 ± 0.24 |
0.60 ± 0.38 |
|
49 |
0.20 ± 0.34 |
0.28 ± 0.48 |
0.38 ± 0.42 |
|
50 |
0.45 ± 0.24 |
0.58 ± 0.10 |
0.64 ± 0.16 |
|
Diclofenac sodium(10mg/kg) |
0.75 ± 0.12 |
0.95 ± 0.12 |
1.16 ± 0.11 |
Experimental Section
Materials and Methods
Chemicals and solvents were purchased from Sigma-Aldrich and Merck. All the reagents were of analytical grade. Thin-layer chromatography (TLC) was performed on E. Merck AL silica gel 60 F254 plates and visualized under UV light. IR spectra were recorded as KBr pellets with Perkin-Elmer Spectrum GX FTIR instrument and only diagnostic and/or intense peaks were reported. 1H NMR spectra were recorded in DMSO- d6 with Varian Mercury plus 400 MHz instrument. Signals due to the residual protonated solvent (1H NMR) served as the internal standard. All the chemical shifts were reported in δ (ppm) using TMS as internal standard. The 1H NMR chemical shifts and coupling constants were determined assuming first-order behavior. Multiplicity was indicated by one or more of the following: s (singlet), d (doublet), t (triplet), q (quartet), m (multiplet), br (broad); the list of coupling constants ( J ) correspond to the order of multiplicity assignment. Mass spectra were recorded with a PE Sciex model API 3000 instrument. All the reactions were carried out under nitrogen atmosphere.
General procedure for the preparation of hydrazone derivatives (39-50)
A mixture of of 4-(1-(2-methoxy-6-pentadecylbenzyl)-1H-1,2,3-triazol-4-yl)benzaldehyde 38 (0.117 mmol) and corresponding substituted phenyl acetohydrazides 11-15 and benzohydrazides 30-36 (0.122 mmol) in ethanol (0.1 mL) was grinded in mortar-pestle for 2-3 min. After completion of the reaction (checked by T.L.C), the precipitated solids were filtered and washed with n-hexane to obtain the corresponding hydrazide-hydrazone derivatives 39-50 in quantitative yields.
(E)-N’-(4-(1-(2-methoxy-6-pentadecylbenzyl)-1H-1,2,3-triazol-4-yl)benzylidene)-2-phenylacetohydrazide (39)
White solid; M.p.: 122-123oC; IR (KBr): υmax 3227 (-CONH, stretching), 1653 (-C=O, stretching), 1608 (-C=N, stretching), 1370 (-C-N, stretching), 1075 (-C-O-C, stretching) cm-1; 1H NMR (300 MHz, DMSO-d6): δ 0.82 (t, J = 6.8 Hz, 3H), 1.35-1.15 (brm, 26H), 2.72 (t, J = 6.9 Hz, 2H), 3.85 (s, 3H), 3.98 (* 3.54, s, 2H), 5.60 (s, 2H), 6.86 (d, J = 8.0 Hz, 1H), 6.94 (d, J = 8.1 Hz, 1H), 7.32-7.23 (broad multiplet, 6H), 7.71 (d, J = 8.1 Hz, 2H), 7.92 (d, J = 7.8 Hz, 2H), 7.99 (s, 1H), 8.35 (*8.21, s, 1H), 11.60 (* 11.38, s, 1H); ESI-MS: m/z, 636.1 (M+H)+.
(E)-N’-(4-(1-(2-methoxy-6-pentadecylbenzyl)-1H-1,2,3-triazol-4-yl)benzylidene)-2-(3,5-dimethylphenyl)acetohydrazide (40)
White solid; M.p.: 106-108oC; IR (KBr): υmax 3188 (-CONH, stretching), 1646 (-C=O, stretching), 1609 (-C=N, stretching), 1371 (-C-N, stretching), 1084 (-C-O-C, stretching) cm-1; 1H NMR (300 MHz, DMSO-d6): δ 0.84 (t, J = 6.4 Hz, 3H), 1.34-1.15 (m, 26H), 2.24 (* 2.21, s, 6H), 2.72 (t, J = 6.9 Hz, 2H), 3.85 (s, 3H), 3.88 (* 3.72, s, 2H), 5.60 (s, 2H), 6.95-6.84 (broad multiplet, 5H), 7.30 (t, J = 8.1 Hz, 1H), 7.70 (d, J = 8.2 Hz, 2H), 7.92 (d, J = 7.8 Hz, 2H), 8.20 (s, 1H), 8.36 (*8.35, s, 1H), 11.50 (* 11.33, s, 1H); ESI-MS: m/z, 664.1 (M+H)+.
(E)-N’-(4-(1-(2-methoxy-6-pentadecylbenzyl)-1H-1,2,3-triazol-4-yl)benzylidene)-2-(2-chloro-4-fluorophenyl)acetohydrazide (41)
Pale yellow solid; M.p.: 96-98oC; IR (KBr): υmax 3199 (-CONH, stretching), 1657 (-C=O, stretching), 1608 (-C=N, stretching), 1370 (-C-N, stretching), 1069 (-C-O-C, stretching) cm-1; 1H NMR (300 MHz, DMSO-d6): δ 0.83 (brs, 3H), 1.35-1.15 (broad multiplet, 26H), 2.72 (t, J = 6.9 Hz, 2H), 3.85 (s, 3H), 4.14 (* 3.72, s, 2H), 5.60 (s, 2H), 6.84 (d, J = 7.2 Hz, 1H), 6.94 (d, J = 7.8 Hz, 1H), 7.49-7.16 (series.multiplet, 4H), 7.71 (d, J = 7.8 Hz, 2H), 7.91 (d, J = 7.8 Hz, 2H), 8.02 (s, 1H), 8.35 (*8.21, s, 1H), 11.64 (* 11.51, s, 1H); ESI-MS: m/z, 688.3 (M+H)+.
(E)-N’-(4-(1-(2-methoxy-6-pentadecylbenzyl)-1H-1,2,3-triazol-4-yl)benzylidene)-2-(4-nitrophenyl)acetohydrazide (42)
Yellow solid; M.p.: 120-121oC; IR (KBr): υmax 3196 (-CONH, stretching), 1651 (-C=O, stretching), 1609 (-C=N, stretching), 1370 (-C-N, stretching), 1088 (-C-O-C, stretching) cm-1; 1H NMR (300 MHz, DMSO-d6): δ 0.83 (t, J = 5.4 Hz, 3H), 1.35-1.15 (m, 26H), 2.73 (t, J = 6.6 Hz, 2H), 3.85 (s, 3H), 4.18 (* 3.74, s, 2H), 5.60 (s, 2H), 6.86 (d, J = 8.1 Hz, 1H), 6.94 (d, J = 8.4 Hz, 1H), 7.30 (t, J = 7.5 Hz, 1H), 7.60 (d, J = 8.1 Hz, 2H), 7.72 (d, J = 8.1 Hz, 2H), 7.92 (d, J = 8.1 Hz, 2H), 8.05 (s, 1H), 8.20 (d, J = 6.9 Hz, 2H), 8.36 (*8.22, s, 1H), 11.69 (* 11.53, s, 1H); ESI-MS: m/z, 681.3 (M+H)+.
(E)-N’-(4-(1-(2-methoxy-6-pentadecylbenzyl)-1H-1,2,3-triazol-4-yl)benzylidene)-2-(2,3-dihydrobenzofuran-5-yl)acetohydrazide (43)
Off-white solid; M.p.: 131-132oC; 1H NMR (300 MHz, DMSO-d6): δ 0.84 (t, J = 6.8 Hz, 3H), 1.35-1.15 (m, 26H), 2.73 (t, J = 6.8 Hz, 2H), 3.15 (t, J = 5.8 Hz, 2H), 3.85 (s, 3H), 3.87 (* 3.50, s, 2H), 4.50 (t, J = 5.7 Hz, 2H), 5.60 (s, 2H), 6.67 (t, J = 8.4 Hz, 1H), 6.86 (d, J = 7.8 Hz, 1H), 6.94 (d, J = 8.7 Hz, 1H), 7.0 (d, J = 8.7 Hz, 1H), 7.15 (s, 1H), 7.32 (t, J = 7.8 Hz, 1H), 7.74 (d, J = 8.1 Hz, 2H), 7.93 (d, J = 8.0 Hz, 2H), 8.35 (*8.20, s, 1H), 11.51 (* 11.31, s, 1H); ESI-MS: m/z, 678.1 (M+H)+.
(E)-N’-(4-(1-(2-methoxy-6-pentadecylbenzyl)-1H-1,2,3-triazol-4-yl)benzylidene)benzohydrazide (44)
off-white solid; M.p.: 108-109oC; IR (KBr): υmax 3437 (-CONH, stretching), 1655 (-C=O, stretching), 1616 (-C=N, stretching), 1368 (-C-N, stretching), 1084 (-C-O-C, stretching) cm-1; 1H NMR (400 MHz, DMSO-d6): δ 0.83 (t, J = 6.8 Hz, 3H), 1.33-1.14 (brm, 26H), 2.71 (t, J = 7.2 Hz, 2H), 3.86 (s, 3H), 5.60 (s, 2H), 6.84 (d, J = 8.0 Hz, 2H), 6.90 (d, J = 8.0 Hz, 2H), 7.06 (d, J = 8.8 Hz, 2H), 7.22 (d, J = 8.0 Hz, 1H), 7.72-7.64 (m, 7H), 7.86 (t, J = 7.2 Hz, 2H), 8.30 (s, 1H), 8.42 (s, 1H), 11.68 (s, 1H); ESI-MS: m/z, 622.4 (M+H)+.
(E)-N’-(4-(1-(2-methoxy-6-pentadecylbenzyl)-1H-1,2,3-triazol-4-yl)benzylidene)-4-methoxybenzohydrazide (45)
off-white solid; M.p.: 131-133oC; IR (KBr): υmax 3286 (-CONH, stretching ),1658 (-C=O, stretching), 1605 (-C=N, stretching),1349 (-C-N, stretching), 1084 (C-O-C, stretching) cm-1; 1H NMR (400 MHz, DMSO-d6): δ 0.81 (t, J = 6.8 Hz, 3H), 1.33-1.14 (brm, 26H), 2.71 (t, J = 7.2 Hz, 2H), 3.84 (s, 6H), 5.60 (s, 2H), 6.85 (d, J = 8.0 Hz, 2H), 6.93 (d, J = 8.0 Hz, 2H), 7.04 (d, J = 8.8 Hz, 2H), 7.28 (t, J = 8.0 Hz, 1H), 7.73-7.68 (m, 2H), 7.90 (t, J = 7.2 Hz, 2H), 8.33 (s, 1H), 8.44 (s, 1H), 11.72 (s, 1H); ESI-MS: m/z, 652.4 (M+H)+.
(E)-N’-(4-(1-(2-methoxy-6-pentadecylbenzyl)-1H-1,2,3-triazol-4-yl)benzylidene)-4-chlorobenzohydrazide (46)
Pale yellow solid; M.p.: 122-123oC; IR (KBr): υmax 3289 (-CONH, stretching ), 1661 (-C=O, stretching), 1602 (-C=N, stretching), 1273 (-C-N, stretching), 1085 (-C-O-C, stretching) cm-1; 1H NMR (400 MHz, DMSO-d6): δ 0.81 (t, J = 6.6 Hz, 3H), 1.33-1.13 (m, 26H), 2.71 (t, J = 7.2 Hz, 2H), 3.84 (s, 3H), 5.60 (s, 2H), 6.83 (d, J = 7.8 Hz, 1H), 6.93 (d, J = 7.8 Hz, 1H), 7.29 (t, J = 7.2 Hz, 1H), 7.58 (d, J = 7.6 Hz, 2H), 7.80 (d, J = 7.6 Hz, 2H), 7.93 (d, J = 8.0 Hz, 3H), 8.38 (s, 1H), 8.44 (s, 1H), 11.85 (s, 1H),; ESI-MS: m/z, 656.3 (M+H)+.
(E)-N’-(4-(1-(2-methoxy-6-pentadecylbenzyl)-1H-1,2,3-triazol-4-yl)benzylidene)-4-fluorobenzohydrazide (47)
Pale yellow solid; M.p.: 128-129oC; 1H NMR (400 MHz, DMSO-d6): δ 0.82 (t, J = 6.6 Hz, 3H), 1.33-1.12 (m, 26H), 2.71 (t, J = 7.2 Hz, 2H), 3.85 (s, 3H), 5.58 (s, 2H), 6.86 (d, J = 7.8 Hz, 1H), 6.95 (d, J = 7.8 Hz, 1H), 7.30 (t, J = 7.2 Hz, 1H), 7.60 (d, J = 7.6 Hz, 2H), 7.78-7.72 (m, 3H), 7.96 (d, J = 8.0 Hz, 3H), 8.36 (s, 1H), 8.48 (s, 1H), 11.88 (s, 1H),; ESI-MS: m/z, 640.4 (M+H)+.
(E)-N’-(4-(1-(2-methoxy-6-pentadecylbenzyl)-1H-1,2,3-triazol-4-yl)benzylidene)-3,4,5-trimethoxybenzohydrazide (48)
off-white solid; M.p.: 117-118oC; IR (KBr): υmax 3228 (-CONH, stretching), 1648 (-C=O, stretching), 1602 (-C=N, stretching), 1335(-C-N, stretching), 1088 (-C-O-C) cm-1; 1H NMR (400 MHz, DMSO-d6): δ 0.80 (t, J = 6.6 Hz, 3H), 1.33-1.12 (brm, 26H), 2.71 (t, J = 7.2 Hz, 2H), 3.71 (s, 3H), 3.84 (s, 3H), 3.83 (s, 6H), 5.58 (s, 2H), 6.84 (d, J = 7.6 Hz, 1H), 6.93 (d, J = 8.4 Hz, 1H), 7.22 (s, 2H), 7.28 (d, J = 8.0 Hz, 1H), 7.75 (d, J = 7.6 Hz, 2H), 7.93 (t, J = 8.0 Hz, 2H), 8.36 (s, 1H), 8.45 (s, 1H), 11.70 (s, 1H); ESI-MS: m/z, 712.4 (M+H)+.
(E)-N’-(4-(1-(2-methoxy-6-pentadecylbenzyl)-1H-1,2,3-triazol-4-yl)benzylidene)-2,5-difluorobenzohydrazide (49)
Yellow solid; M.p.: 130-131oC; IR (KBr): υmax 3252 (-CONH, stretching), 1655 (-C=O, stretching), 1603 (-C=N, stretching), 1363 (-C-N, stretching), 1085 (C-O-C, stretching) cm-1; 1H NMR (400 MHz, DMSO-d6): δ 0.81 (t, J = 7.2 Hz, 3H), 1.36-1.15 (m, 26H), 2.72 (t, J = 7.2 Hz, 2H), 3.84 (* 3.83, s, 3H), 5.59 (* 5.56, s, 2H), 6.85 (d, J = 7.2 Hz, 1H), 6.93 (d, J = 7.6 Hz, 1H), 7.50-7.29 (m, 5H), 7.81 (* 7.75, d, J = 8.0 Hz, 2H), 7.92 (d, J = 7.6 Hz, 2H), 8.25 (* 8.06, s, 1H), 8.32 (s, 1H), 11.99 (* 11.81, s, 1H); ESI-MS: m/z, 657.8 (M+H)+.
(E)-N’-(4-(1-(2-methoxy-6-pentadecylbenzyl)-1H-1,2,3-triazol-4-yl)benzylidene)-3-nitrobenzohydrazide (50)
Yellow solid; M.p.: 100-101oC; IR (KBr): υmax 3232 (-CONH, stretching), 1656 (-C=O, stretching), 1613 (-C=N, stretching), 1348 (-C-N, stretching), 1083 (C-O-C, stretching) cm-1; 1H NMR (400 MHz, DMSO-d6): δ 0.80 (t, J = 7.2 Hz, 3H), 1.34-1.12 (brm, 26H), 2.71 (t, J = 7.2 Hz, 2H), 3.84 (s, 6H), 5.60 (s, 2H), 6.85 (d, J = 8.2 Hz, 1H), 6.94 (d, J = 8.2 Hz, 1H), 7.29 (t, J = 8.2 Hz, 1H), 7.78 (d, J = 8.2 Hz, 2H), 7.84 (t, J = 8.0 Hz, 1H), 7.98 (d, J = 8.2 Hz, 2H), 8.50-8.3 (m, 4H), 8.74 (s, 1H), 12.14 (s, 1H); ESI-MS: m/z, 667.4 (M+H)+.
Biology Experimental
Ant-inflammatory Activity
A standard model system, carrageenan induced inflammatory rat model was followed for the experimentation on acute inflammatory conditions reported by us recently 45, 46.
Conclusion
The present paper describes the synthesis of some new hydrazide-hydrazone derivatives 39-50 (linked with anacardic acid and 1,2,3-triazole ring) and evaluated for anti-inflammatory activity. Compounds 41 and 43 bearing 2-phenylacetohydrazides with substitution R = 2-Cl-4-Fluoro and tetrahydrofuran ring exhibited excellent anti-inflammatory activity and compounds 39, 40 and 42 (bearing 2-phenylacetohydrazides with substitution R = H, 3,5-CH3 and 4-NO2) displayed good anti-inflammatory activity.
Acknowledgements
One of the author (A.L.V) thanks Dr.B.Vasudha, Department of Pharmacy, Lalitha College of Pharmacy, Ghatkesar, Hyderabad for her help in carrying out the antimicrobial studies.
References
- Swamy, P.V.; Chandrasekhar, K.B.; P. C. Kambhampati. J. Applicable Chem. 2015, 4, 492.
- Rathore, K.S.; Jadon, G. J. Drug Delivery Ther. 2014, 4, 131.
- Asif, M.; Husain, A. J. Appl. Chem. 2013, ID 247203.
- Lacerda, R.B.; da Silva, L.L.; de Lima, C.K.F.; Miguez, E.; Miranda, A.L.P.; Laufer, S. A. PLoS one., 2012, 7, 1-12.
CrossRef - Kumar, A.; Verma, A.; Chawla, G.; Vaishali. Int. J.ChemTech Res. 2009, 1, 1177.
- Kaplancikli, Z.A.; Altintop, M.D.; Özdemir, A.; Turan-Zitouni, G.; Khan, S.I.; Tabanca, N. Lett. Drug Des.Dis. 2012, 9, 310.
CrossRef - Mohareb, R.M.; El-Sharkawy, K.A.; Hussein, M.M.; El-Sehrawi, H.M. J. Pharm. Sci. Res. 2010, 2, 185.
- Koopaei, M.N.; Assarzadeh, M.J.; Almasirad, A.; Ghasemi-Nirib, S.F.; Amini, M.; Kebriaeezadeh, A. Iran J.Pharm. Res. 2013, 12, 721.
- Kumar, N.; Chauhan, L.S.; Dashora, N.; Sharma, C.S. Sch. Acad. J. Pharm. 2014, 3, 366.
- Mohammad, A. Int. J. Chem. Appl. Biol. Sci. 2014, 1, 23.
CrossRef - Wang, H.; Ren, S.X.; He, Z.Y.; Wang, D.L.; Yan, X.N.; Feng, J.T. Int. J. Mol. Sci. 2014, 15, 4257.
CrossRef - Ibrahim, N.M.; Yosef, H.A.A.; Ewies, E.F.; Mahran, M.R.H.; Ali, M.M.; Mahmoud, A.E. J. Braz. Chem. Soc. 2015, 26,1086.
- Potu°cˇkova´, E.; Hrusˇkova´, K.; Buresˇ, J.; Kovarˇı´kova´, P.; pirkova´, I.A.S.; Pravdı´kova´, K. PLoS ONE. 2014, 9, 1.
- Sharma, M.; Chauhan, K.; Srivastava, R.K.; Singh, S.V.; Srivastava, K.; Saxena, J.K. Chem. Biol.Drug.Des. 2014, 84, 2, 175.
- Asif, M. Int. J. Adv. Chem. 2014, 2, 85.
- Khanmohammadia, H.; Mohammad, H.A.; Hosseinzadeh, A.; Erfantalab , M. Spectrchim. Acta A. 2008, 71, 1474.
CrossRef - Singh, K.; Barwam, M.S.; Tyagi, P. Eur. J. Med. Chem. 2006, 41, 147.
CrossRef - Liu, K.; Shi, W.; Cheng, P. Dalton Trans. 2011, 40, 8475.
CrossRef - Bascal, Z.; Holden-Dye, L.; Willis, R.J.; Smith, S.W.G.; Walker, R.J. Parasitology. 1996, 112, 253.
CrossRef - Biagi, G.; Giorgi, I.; Livi , O.; Lucacchini, A.; Martini, C.; Scartoni, V.J. Pharm Sci. 1993, 82, 893.
CrossRef - Moltzen, E.K.; Pedersen, H.; Bogeso, K.P.; Meier, E.; Frederiksen, K.; Sanchez, C.; Lembol, K.L. J Med Chem.1994, 37, 4085.
CrossRef - Olesen, P.H.; Sorensen, A.R.; Urso, B.; Kurtzhals, P.; Bowler, A.N.; Ehrbar, U.; Hansen, B.F. J. Med. Chem. 2003, 46, 3333.
CrossRef - Chakrabarti, J.K.; Hotten, T.M.; Pullar, I.A.; Steggles, D.J. J. Med Chem. 1989, 32, 2375.
CrossRef - Alvarez, R.; Velazquez, S.; San-Felix, A.; Aquaro, A.; De Clercq, E.; Perno, C.F.; Karlsson, A.; Balzarini, J.; Camarasa, M.J.; J Med Chem. 1994, 37, 4185.
CrossRef - Sanghvi, Y.S.; Bhattacharya, B.K.; Kini, G.D.; Matsumoto, S.S.; Larson, S.B.; Jolley, W.B.; Robins, R.K.; Revankar, G.R. J Med Chem. 1990, 33, 336.
CrossRef - Buckle, D.R.; Rockell, C.J.M.; Smith, H.; Spicer, B.A. J Med Chem. 1986, 29, 2262.
CrossRef - Hupe, D.J.; Boltz, R.; Cohen, C.J.; Felix, J.; Ham, E.; Miller, D.; Soderman, D.; Van Skiver, D. J Biol Chem. 1991, 266, 10136.
- Guda, D.; Wang, T.; Cho, H.M.; Lee, E.; Tetrahedron Letters. 2012, 53, 5238.
CrossRef - Yang, Y.; Zhang, X.; Hong, J. Trans.Met.Chem. 2009, 34, 791.
- Nasser, S.; Khalil, A.; Eur. J. Med. Chem. 2010, 45, 5265.
CrossRef
- Subashchandrabose, S.; Krishnan, A.R.; Saleem, H.; Parameswari, R.; Sundaraganesan, N.; Thanikachalam , V.; Manikandan, G. Spectrochim. Acta, Part A. 2010, 77, 877.
CrossRef - Bermejo, E.; Carballo, R.; Castineiras, A.; Dominguez, R.; Maichle-Mossmer, C.; Strahle, J.; West, D. Polyhedron. 1999, 18, 3695.
CrossRef - Almajan, G.L.; Barbuceanu, S.F.; Almajan, E.R.; Draghici, C.; Saramet, G. Eur. J. Med. Chem. 2008, 43, 155.
CrossRef - Ma, L.Y.; Pang, L.P.; Wang, B.; Zhang, M.; Hu, B.; Xue, Shao, K.P.; Zhang, B.L.; Liu E. Zhang, Y.; Liu, H.M. Eur. J. Med. Chem. 2014, 86, 368.
CrossRef - Murata, M.; Irie, J.; Homma, S.; Lebensm Wiss Technol. 1997, 30, 458.
CrossRef - Grazzini, R.; Hesk, D.; Heininger, E. Biochem Biophys Res Commun. 1991, 176, 775.
CrossRef - Kishore, A.H.; Vedamurthy, B.M.; Mantelingu, K. J Med Chem. 2008, 28, 792.
CrossRef - Sun, Y.; Jiang, X.; Chen, S.; Price, B.D. FEBS Lett. 2006, 580, 4353.
CrossRef - Sung, B.; Pandey, M.K.; Ahn, K.S.; Yi, T.; Chaturvedi, M.M.; Liu, M.; Aggarwal, B.B.; Blood. 2008, 111, 4880.
CrossRef - Choi, J.G.; Jeong, S.I.; Ku, C.S.; Sathishkumar, M.; Lee, J.J.; Mun, S.P.; Kim, S.M. Fitoterapia. 2009, 80, 18.
CrossRef - Sukumari-Ramesh, S.; Singh, N.; Jensen, M.A.; Dhandapani, K.M.; Vender, J.R. J Neurosurg. 2011, 114, 1681.
CrossRef - Rea, A.I..; Schmidt, J.M.; Setzer, W.N.; Sibanda, S.; Taylor, C.; Gwebu, E.T. Fitoterapia. 2003, 74, 732.
CrossRef - Sullivan, J.T.; Richards, C.S.; Lloyd, H.A.; Krishna, G. Planta Med. 1982, 44, 175.
CrossRef - Trevisan, M.T.S.; Pfundstein, B.; Haubner, R.; Würtele, G.; Spiegelhalder, B.; Bartsch, H.; Owen, R.W.; Chem. Food Toxicol. 2006, 44, 188.
CrossRef - Winter, C.A.; Risley, E.A.; Nuss, G.W. Proc. Soc. Exp. BiMol. ed. 1962, 111, 544.
- Kumar Reddy, A.L.V.; Niren E Kathale. Orient.J.Chem. 2017, 33, 971.
- Kumar Reddy, A.L.V.; Niren E Kathale. J.Applicable.Chem. 2016, 5, 783.
- Sarbani Pal et al. J. Chem. Pharm. Res. 2010, 2, 393.
- Palmer, R.B.; Andersen, N.H. Bioorg. Med. Chem. Lett., 1996, 6, 2173.
CrossRef - Palla, G.; Predieri, G.; Domiano, P. Tetrahedron. 1986, 42, 3649.
CrossRef

This work is licensed under a Creative Commons Attribution 4.0 International License.









