Solvent-free Isomerization of 3-carene to 2-carene using Na/o-chlorotoluene Catalyst in trans-isolimonene Production
Tatang Shabur Julianto1,3 , Jumina1, Hardjono Sastrohamidjojo1 and Mustofa2
, Jumina1, Hardjono Sastrohamidjojo1 and Mustofa2
1Doctoral Program, Department of Chemistry, Faculty of Mathematics and Natural Sciences, Gadjah Mada University, Jalan Sekip Utara, Yogyakarta, Indonesia.
2Pharmacology and Therapy Unit, Department of Medicine, Gadjah Mada University, Jalan Sekip Utara, Yogyakarta, Indonesia.
3Department of Chemistry, Faculty of Mathematics and Natural Sciences, Universitas Islam Indonesia, Jalan Kaliurang Km.14,5 Yogyakarta, Indonesia.
Corresponding Author E-mail: tatang_shabur@uii.ac.id
DOI : http://dx.doi.org/10.13005/ojc/330652
Trans-isolimonene is one of the important compound in drugs development. This compound can be made by isomerization of inexpensive 3-carene via 2-carene using Na/o-chlorotoluene catalyst in xylene. The existence of xylene as a solvent requires a further separation process that can reduce the efficiency when applied in the bulk industry. The isomerization of 3-carene to 2-carene has been studied in solvent free reaction compared by isomerization without xylene solvent. The result showed that the isomerization can also occur in solvent-free condition. Solvent-free isomerization gave 27.72% of conversion and 83.27% of selectivity while isomerization with solvent gave 23.59% of conversion and 86.87% of selectivity.
KEYWORDS:3-carene; 2-carene; Na/o-chlorotoluene; Xylene; Trans-isolimonene; Isomerization
Download this article as:| Copy the following to cite this article: Julianto T. S, Jumina J, Sastrohamidjojo H, Mustofa M. Solvent-free Isomerization of 3-carene to 2-carene using Na/o-chlorotoluene Catalyst in trans-isolimonene Production. Orient J Chem 2017;33(6). |
| Copy the following to cite this URL: Julianto T. S, Jumina J, Sastrohamidjojo H, Mustofa M. Solvent-free Isomerization of 3-carene to 2-carene using Na/o-chlorotoluene Catalyst in trans-isolimonene Production. Orient J Chem 2017;33(6). Available from: http://www.orientjchem.org/?p=39848 |
Introduction
Trans-isolimonene is one of monoterpenoid compounds that a rare and little-studied substance. It was first synthesized by Tshugaev in 1903 1. Now trans-isolimonene may become a fully accessible monoterpenoid use as one of starting material for some drug compounds development such as in synthesis of antimalarial drug artemisinin 2 and synthesis of menthol 3. This compound is rarely found naturally in plants. Several studies have reported that isolimonene found in essential oils of Citrus medica and Citrus reticulate. The peel oil of Citrus medica contain isolimonene about 39.37% 4,5 and 5.87% in Citrus reticulate essential oil 6. The importance of trans-isolimonene compounds in drugs development not comparable with the availability in nature, therefore sought other methods for producing trans-isolimonene using synthesis way.
Trans-isolimonene can be produced from 2-carene via thermal isomerization, while compound 2-carene was obtained using isomerization of 3-carene. The isomerization of 3-carene to 2-carene is reported to take place in several methods. Over hydrogenation catalysts such as Raney nickel and Pd on carbon, 2-carene was produced in addition to the hydrogenated products under a hydrogen atmosphere but the selectivity of this reactions is low because of hydrogenation to carane 7. Shimazu, et al. 8 reported that 3-carene undergoes isomerization to 2-carene over solid bases of MgO and CaO, Ni/SiO2 catalyst 9, glaucolite 10, and basic zeolite 11. All of these previous methods need high temperature condition and low selectivity of 2-carene.
In this work, we prepared 2-carene from 3-carene using Booth’s method 12. The reaction occur at certain temperature with xylene solvent and catalyst Na/o-chlorotoluene for 1 night. The existence of xylene solvent requires a separation process using fractional distillation to separate the product from the solvent.
In this study, our objective is to understand the influence of solvent-free isomerization 3-carene to 2-carene on its conversion and selectivity.
Materials and Method
Materials
Sodium metal, 3-carene, and o-chlorotoluene were purchased from Merck-Millipore (Germany). Analysis by using Gas Chromatography-Mass Spectrometer (GC-MS) Shimadzu QP2100SP with RTX-5 column.
Isomerisation of 3-carene to 2-carene
With Solvent of Xylene
A total of 15 mg 3-carene included in the reflux of 500 mL flask along with 15 mL xylene and 0.5 g of sodium metal and 0.5 mL of o-chlorotoluene. The mixture refluxed for 12 hours, 24 hours, and 48 hours respectively. Furthermore, reflux resulting solution is cooled to room temperature. The solution was decanted to separate the catalyst from the solution. Then the solution was analyzed using Gas Chromatography – Mass Spectrometer to determine the equilibrium formation of 3-carene and 2-carene 12.
Without Solvent of Xylene
A total of 30 mL 3-carene included in the reflux of 500 mL flask along with 0.5 g of sodium metal catalyst and 0.5 mL o-chlorotoluene. The mixture refluxed for 12, 24, and 48 hours respectively. Furthermore, reflux resulting solution is cooled to room temperature. The solution was decanted to separate the catalyst from the solution. Then the solution was analyzed using Gas Chromatography–Mass Spectrometer to determine the equilibrium formation of 3-carene and 2-carene.
Result and Discussion
Influence of Solvent free Condition on Isomerization 3-carene to 2-carene
The isomerization of 3-carene to 2-carene can be done using alkaline catalyst of sodium/o-chlorotoluene. Reaction between sodium metal and o-chlorotoluene produces a salt-containing carbanion act as strong base (Scheme 1).
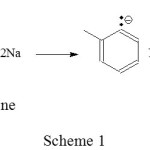 |
Scheme 1 Click here to View scheme |
Furthermore, this carbanion take hydrogen atoms on saturated C-C bond of 3-carene causing electron rearrangement to form 2-carene isomer (Scheme 2).
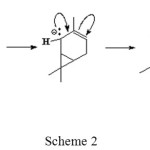 |
Scheme 2 Click here to View scheme |
Reaction mechanism that is catalyzed olefin isomerization base is closely related to the chemical nature of the carbanion. Reaction can occur in a homogeneous solution system and the presence of a strong base catalyst. In the previous study, the solvent xylene used to support the catalyst basicity. The solvent sufficiently suitable to induce the formation of carbanion.
This study has been conducted isomerization of 3-carene to 2-carene without the use of solvent compared to the isomerization with solvent condition. Our objective in this study was to avoid the using solvent in this isomerization reaction. Figure 1 showed the results of isomerization reaction with solvent xylene and without solvent condition. Both of reactions refluxed for 16 hours.
 |
Figure 1: Chromatograms of isomerization result of 3-carene refluxed for 16 hours (A) with xylene solvent (B) without solvent Click here to View figure |
Refluxing for 16 hours did not gave 2-carene product. It need more time to optimize isomerization condition. Addition of reflux time have been observed. Figure 2 demonstrated the result of isomerization for 24 hours of refluxing. Solvent-free isomerization also gave product of 2-carene as well as reaction with xylene solvent. At temperature 150-170°C the thermodynamic equilibrium between 3-carene and 2-carene corresponds to a mixture of 60% of 3-carene and 40% of 2-carene.
 |
Figure 2: Chromatograms of isomerization result of 3-carene refluxed for 24 hours (A) with xylene solvent (B) without solvent Click here to View figure |
Figure 3 showed that more time refluxing for 48 hours also gave 2-carene product but at the same time obtained by-products of m-cymene and p-cymene.
 |
Figure 3: Chromatograms of isomerization result of 3-carene refluxed for 48 hours (A) with xylene solvent (B) without solvent Click here to View figure |
According to previous research, cymene can obtained through dehydrogenation 3-carene and 2-carene (scheme 3) 9.
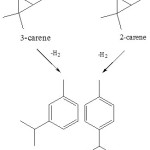 |
Scheme 3 Click here to View scheme |
These results showed that the isomerization also occur in solvent-free condition. The optimum result according to this work is the isomerization for 24 hours, while the isomerization for 48 hours obtained by-products of cymenes.
Conversion and Selectivity of Isomerization 3-carene to 2-carene
Conversion and selectivity were measured based on amount of chromatograms area. The conversion of 3-carene (C), selectivity of 2-carene (S) are described by the following equations that [3c]o represents the initial concentration of 3-carene and [3c]t and [2c]t represent the final concentration of 3-carene and 2-carene, respectively.
C = ([3c]o – [3c]t )/[3c]o
S = [2c]t / ([3c]o – [3c]t)
Conversion of 3-carene in both of condition increased with increasing reaction time (Figure 4).
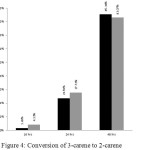 |
Figure 4: Conversion of 3-carene to 2-carene Click here to View fgiure |
The optimum isomerization selectivity of 3-carene to 2-carene reached when the reaction conducted in 24 hours refluxing. The selectivity of isomerization with xylene solvent and isomerization without solvent are 86.87% and 83.27%, respectively (Figure 5). There were no significant differences between xylene solvent and without solvent condition. It reinforces previous research that isomerization of 3-carene to 2-carene can also be done without using solvent as well as using xylene solvent.
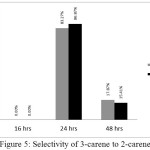 |
Figure 5: Selectivity of 3-carene to 2-carene Click here to View figure |
Conclusion
In conclusion, the free solvent isomerization of 3-carene to 2-carene can be done for 24 hours with 27.72% of conversion and 83.27% of selectivity.
Acknowledgments
This research was financially supported by Scholarship of Indonesian Directorate of Higher Education (DIKTI) and Directorate of Research and Community Service of Islamic University of Indonesia (UII).
References
- Beilstein. 1904. J. Russ. Phys. Chem. Soc. 36 in Sutherland, Maurice, D. A. Review of Densities and Refractive Indices of The Terpene. Papers. Department of Chemistry, University of Queensland. 1948, (1)34, 11
- Yadav, J.S., Babu, R.S., and Sabitha, G. Total Synthesis of Artemisinin. ARKIVOC, 3, 2003, 125
- Lawrence, B.M. Mint-The Genus Mentha: Medicinal and Aromatic Plant-Industrial Profile. CRC Press, Boca Raton, 2007, 388
- Bhuiyan, M.N.I.; Begum, J.; Sardar, P.K.; and Rahman, M.S.; Constituents of Peel and Leaf Essential Oils of Citrus medica L. J. Sci. Res., 2009, 1 (2) 387-392
- Meena A.K.; Kandale Ajit; Rao A.M. A Review on Citron-Pharmacognosy, Phytochemistry and Medicinal Uses. IRJP, 2011, 2 (1), 14-19
- Fayed, S. A. Antioxidant and Anticancer Activities of Citrus reticulate (Petitgrain Mandarin) and Pelargonium graveolens (Geranium) Essential Oils. Res. J. Agric. & Biol. Sci., 2009, 5(5): 740-747
- Basset, J. M. Modern Surface Organometallic Chemistry, Wiley-VCH, Weinheim, 2006, 124
- Shimazu, K.; Hattori, H; Tanabe, K. Isomerization of 3-carene over MgO and CaO, Journal of Catalysis. 1977, 48, 302-311
CrossRef - Lesage, P., Candy, J. P., Hirigoyen, C., Humblot, F., Leconte, M., Basset, J. M., An example of the valorization of terpenes: The selective isomerization of 3-carene to 2-carene in the presence of silica supported nickel catalysts modified by tetrabutyl tin. J. Mol. Cat. A: Chemical 112, 1996, 303-309
CrossRef - Utenkova, D. B.; Skakovskii, E. D.; Senkov, G. M.; Agabekov, V. E.; Baranovskii, A. V.; Bogushevich, S. E.; and. Sidorenko, A. Y. NMR and GC analysis of 3-carene Isomerization over Activated Glauconite, Journal of Spectroscopy, 2017, 83(6), 1026-1030
- Meyer, U., and Hoelderich, W.F. Application of basic zeolites in the decomposition reaction of 2-methyl-3-butyn-2-ol and the isomerization of 3-carene. Journal of Molecular Catalysis A: Chemical 142, 1999, 213–222
CrossRef - Booth, A. B. and Island, J. 8-halo-2-menthene and its method of preparation. United States Patent Office, August 2, 1973, 3755472

This work is licensed under a Creative Commons Attribution 4.0 International License.









