Selective Naked-eye Detection of Acetate by New Azo-Isonicotinohydrazide Derivatives
Sajjad Q. Makki1 and Asim A. Balakit2
and Asim A. Balakit2
1Department of Chemistry, College of Education, University of Al-Qadisiyah, Iraq.
2College of Pharmacy, University of Babylon, Babylon, Iraq.
Corresponding Author E-mail: asim_alsalehi@hotmail.com
DOI : http://dx.doi.org/10.13005/ojc/330659
Three new hydrazone, based on azo-isonicotinohydrazide derivatives, have been designed, synthesized and characterized by FT-IR, 1H NMR and 13C NMR spectroscopy. The new compounds were designed to act as selective molecular sensors for naked-eye detection of acetate anion. To study the effect of the substituent at certain position in the main structure on the selectivity of the sensor, the compounds were designed to have –CH3, –H or –NO2 at that position. Solutions of different anions (F−, H2PO4−, NO2−, NO3−, Cl−, HSO4−) and AcO− were used to test the selectivity. The results revealed that the compound with electron withdrawing group (NO2) interacts selectively with acetate producing a significant feasible color change which makes it considered as selective molecular sensor for naked-eye detection of acetate. The dissociation constant for AcO− with the new sensor was also determined.
KEYWORDS:Isonicotinohydrazide; Hydrazones; Azo; Schiff; Anions; Acetate; Sensors
Download this article as:| Copy the following to cite this article: Makki S. Q, Balakit A. A. Selective Naked-eye Detection of Acetate by New Azo-Isonicotinohydrazide Derivatives. Orient J Chem 2017;33(6). |
| Copy the following to cite this URL: Makki S. Q, Balakit A. A. Selective Naked-eye Detection of Acetate by New Azo-Isonicotinohydrazide Derivatives. Orient J Chem 2017;33(6). Available from: http://www.orientjchem.org/?p=40254 |
Introduction
Anions are widespread species and play substantial roles in environmental, biological and ecosystems; therefore, their recognition and sensing have attracted a considerable attention. (1-6) The molecular sensing of anions is one of the most important topics in supramolecular chemistry (7-10), the ability of the molecular sensor to form non-covalent interactions, particularly selective hydrogen bonding interactions with the analytes is fundamental and crucial point based on which molecular sensors are designed. Molecules that containing functional groups such as amide (11-14), urea (15-16) and hydroxyl (17) have been openly used to sense anions by hydrogen bonding interactions for numerous important anionic analytes(18), on other hand, Schiff bases have been utilized to develop chemosensors with selective sensitivity to anions, especially ones containing phenolic groups in their structures are used in one of strategies of designing sensor for anions due to the ability of the phenolic –OH group to interact with anions by hydrogen bonding.(19) Among the various anions, acetate has attracted researcher to develop molecular sensors to detect it selectively, acetate performs important roles in organic, environmental and biological processes such as biocatalysis, antibodies and other cellular processes. (20-22) Furthermore, acetate is taken into consideration as an indicator of natural decomposition in marine sediments. (23)
There is a growing interest in the development of small molecules that used for colorimetric sensing which allows naked-eye detection of the analytes without going to any sophisticated and expensive techniques. (24) Here we introduce a novel hydrazone based molecular sensors derived form isonicotinohydrazide for selective naked-eye detection of acetate.
Materials and Methods
All reagents and chemicals were obtained from commercial suppliers and used without further purification. Solutions of anions were prepared from their sodium salts. 1H, 13C NMR spectra were recorded on a Bruker Avance III spectrometer at 400 MHz using DMSO-d6 with TMS reference. FT-IR spectra were recorded on Tensor II, Bruker-Optics FT-IR spectrophotometer. UV–vis spectra were recorded on a Shimadzu UV-1800 spectrophotometer with quartz cuvettes (path length = 1 cm) at room temperature.
Synthesis
Synthesis of the Azo Compounds (1a–3a)
Compounds 1a-3a were synthesized according to literature procedure. (25) To a solution of aniline derivative (4 mmol) in water (2 mL), concentrated hydrochloric acid (8 mL) was added gently with stirring. The solution was cooled down to 0-5°C in ice bath, diazotized with solution of sodium nitrite (0.315 gm, 4.5 mmol) in water (3.5 mL), at this low temperature.
The cold solution of the produced diazonium salt was added dropwise to a solution of salicyladehyde (0.479 ml, 4 mmol) in (20 mL) of water including sodium carbonate (2.92 g, 27.55 mmol) and sodium hydroxide (0.160 g, 4 mmol) at 0-5°C. The reaction mixture was stirred for one hour in the ice bath, then allowed to warm up gently to the room temperature. The product was collected by filtration and washed with ethanol.
2-Hydroxy-5-(phenyldiazenyl)benzaldehyde (1a)
Yield = 55%. MP = 128-129°C, FT-IR (KBr, cm-1): ν 3208 (OH), 1668 (C=O), 1574 (N=N); 1621 (C=C Aromatic). 1H NMR (400 MHz, DMSO-d6) δ 11.66 (s, 1H, OH), 10.41 (s, 1H, CHO), 8.21 (s, 1H, H-Aromatic), 8.13 (d, J = 8.8 Hz, 1H, H-Aromatic), 7.90 (d, J = 7.2 Hz, 2H, H-Aromatic), 7.60 (dt, J = 12.3, 6.8 Hz, 3H, H-Aromatic), 7.23 (d, J = 8.9 Hz, 1H, H-Aromatic) 13C NMR (100 MHz, DMSO-d6) δ 191.0, 164.3, 152.4, 145.1, 131.6, 130.1, 129.9, 124.3, 123.1, 122.8, 119.1.
2-Hydroxy-5-(p-tolyldiazenyl)benzaldehyde (2a)
Yield = 68%. MP = 153-154°C, FT-IR (KBr, cm-1): ν 3189 (OH), 1660 (C=O), 1570 (N=N); 1618 (C=C Aromatic) 1H NMR (400 MHz, DMSO-d6) δ 11.62 (s, 1H, OH), 10.40 (s, 1H, CHO), 8.18 (s, 1H, H-Aromatic), 8.10 (d, J = 8.9 Hz, 1H, H-Aromatic), 7.81 (d, J = 8.2 Hz, 2H, H-Aromatic), 7.42 (d, J = 8.2 Hz, 2H, H-Aromatic), 7.21 (d, J = 8.9 Hz, 1H, H-Aromatic), 2.43 (s, 3H, CH3). 13C NMR (100 MHz, DMSO-d6) δ 191.1, 164.1, 150.4, 145.1, 141.7, 130.4, 130.1, 124.1, 123.1, 122.8, 119.1, 21.5.
2-Hydroxy-5-[(4-nitrophenyl)diazenyl]benzaldehyde (3a)
Yield = 62%. MP = 206-208°C, FT-IR (KBr, cm-1): ν 3114(OH), 2225 (C≡N), 1662 (C=O), 1573 (N=N); 1619 C=C Aromatic) 1H NMR (400 MHz, DMSO-d6) δ 11.83 (s, 1H, OH), 10.41 (s, 1H, CHO), 8.45 (d, J = 7.4 Hz, 2H, H-Aromatic), 8.27 (s, 1H, H-Aromatic), 8.18 (d, J = 2.2 Hz, 1H, H-Aromatic), 8.08 (d, J = 7.8 Hz, 2H, H-Aromatic), 7.27 (d, J = 8.9 Hz, 1H, H-Aromatic).13C NMR (100 MHz, DMSO-d6) δ 190.7, 164.7, 154.5, 145.2, 134.3, 130.5, 125.0, 123.5, 123.2, 119.1, 119.0, 113.3.
Synthesis of azo-Isonicotinohydrazide derivatives (1b–3b)
In 50 mL round bottomed flask charged with magnetic bar and equipped with condenser, isonicotinic hydrazide (0.549 gm, 4 mmol) and appropriate compound (1a-3a; 4 mmol) were mixed and dissolved in a mixture of CHCl3:Ethanol (5:15), four drops of concentrated sulfuric acid were then added. The reaction mixture was stirred under reflux conditions for 4 hours. The mixture was cooled down to room temperature, then, the formed precipitate were collected by filtration and washed with ethanol and chloforom, respectively. (26)
N’-(2-Hydroxy-5-[(phenyldiazenyl)benzylidene]isonicotinohydrazide (1b)
Yield = 92%. MP = 299-300°C, FT-IR (KBr, cm-1): ν 3175 (OH & NH), 1655 (C=O), 1604 (C=N), 1544 (N=N); 1468 (C-N amide), 1H NMR (400 MHz, DMSO-d6) δ 12.42 (s, 1H, NH), 11.68 (s, 1H, OH), 8.85 (d, J = 5.6 Hz, 3H, CH and H-Aromatic), 8.34 (d, J = 2.5 Hz, 1H, H-Aromatic), 8.00–7.87 (m, 5H, H-Aromatic), 7.67–7.52 (m, 3H, H-Aromatic), 7.18 (d, J = 8.8 Hz, 1H, H-Aromatic). 13C NMR (100 MHz, DMSO-d6) δ 162.0, 160.6, 152.5, 150.9, 147.4, 145.6, 140.5, 131.4, 129.9, 126.8, 123.3, 122.8, 122.0, 120.4, 117.8.
N’-(2-Hydroxy-5-[p-tolyldiazenyl)benzylidene]isonicotinohydrazide (2b)
Yield = 93%, MP = 271-272°C, FT-IR (KBr, cm-1): ν 3171 (OH & NH), 1652 (C=O), 1601 (C=N), 1546 (N=N); 1460 (C-N amide), 1580 (C=C Aromatic), 1H NMR (400 MHz, DMSO-d6) δ 12.17 (s, 1H, NH), 11.71 (s, 1H, OH), 8.85 (m, 3H, CH and H-Aromatic), 8.30 (d, J = 2.5 Hz, 1H, H-Aromatic), 7.95–7.90 (m, 3H, H-Aromatic), 7.83 (d, J = 8.0 Hz, 2H, H-Aromatic), 7.42 (d, J = 8.0 Hz, 2H, H-Aromatic), 7.17 (d, J = 8.8 Hz, 1H, H-Aromatic), 2.44 (s, 3H, CH3). 13C NMR (100 MHz, DMSO-d6) δ 162.0, 160.5, 157.5, 150.9, 150.6, 147.5, 145.6, 141.5, 140.5, 130.4, 126.7, 122.8, 122.0, 120.3, 117.8, 21.5.
N’-(2-Hydroxy-5-[(4-nitrophenyl)diazenyl)benzylidene]isonicotinohydrazide (3b)
Yield = 92%, MP = 310-311°C, FT-IR (KBr, cm-1): ν 3201 (OH & NH), 1651 (C=O), 1604 (C=N), 1521 (N=N); 1146 (C-O), 1549 (C=C Aromatic), 1458 and 1340 (NO2). 1H NMR (400 MHz, DMSO-d6) δ 12.44 (s, 1H, NH), 11.89 (s, 1H, OH), 8.85 (m, 3H, CH and H-Aromatic), 8.52–8.38 (m, 3H, H-Aromatic), 8.15–8.07 (m, 2H, H-Aromatic), 8.02 (d, J = 8.8 Hz, 1H, H-Aromatic), 7.94–7.85 (m, 2H, H-Aromatic), 7.21 (d, J = 8.8 Hz, 1H, H-Aromatic). 13C NMR (101 MHz, DMSO-d6) δ 162.0, 161.8, 155.9, 150.9, 148.5, 147.0, 145.7, 140.5, 127.5, 125.5, 1240, 123.7, 122.0, 120.7, 118.0.
Spectrophotometric Studies
For the spectrophotometric studies, 1.35*10−3 M solutions of the compounds 1-3 and 1.35*10−3 M solutions of sodium salts of the anions (F−, H2PO4−, NO2−, NO3−, Cl−, HSO4− and AcO−) in (9:1, DMSO:H2O) were prepared and freshly used at room temperature.
To determine the dissociation constant, the absorption data were fitted to the equation (1):
–log [A−] =log βA− + log[(Amax − A)/(A − Amin)] …………………………………………….(1)
Where log [A−] is the logarithm molar anion concentration at a certain point, log βA− is the dissociation constant, Amax is the maximum absorbance at the selected wavelength, Amin is the minimum absorbance at the same wavelength and A is the observed absorbance at that specific wavelength. Plotting log [(Amax − A)/(A − Amin)] versus the −log [A−], the log βA− was derived from the slope of the resulting plot.(27-28)
Results and Discussion
The new compounds 1b–3b were designed to have binding site that capable of doing hydrogen bonding with the acetate. The signaling unit of the molecule is designed as azo moiety with different substituents; electron donating group (–CH3), electron with drawing group (–NO2) and neutral –H at the para position to the –N=N– linkage, this is expected to cause different effects on the electronic properties of the chromophore and allow to study and proof the mechanism of interaction and the selectivity of molecular sensor toward acetate. The three new compounds 1b–3b were synthesized by two steps synthetic pathway, first three azo aldehydes 1a–3a were prepared, the reason for choosing these compounds (1a–3a) is beside the desired electronic properties of them as azo compounds (good signaling units); they have aldehyde group which is easily condensed with the free amino group of the isonicotinic hydrazide via Schiff base chemistry producing part of the molecule (binding site) can interact with the acetate through the hydrogen bonding where it has free –OH, –NH groups in a configuration that allow the molecule to interact selectively with acetate to form a stable complex with observable color change.
In the second step, 1a–3a were used for the synthesis of the target molecular sensors via Schiff base chemistry when they condensed with isonicotinic hydrazide, Scheme 1. The novel compounds were characterized by FT-IR, 1H NMR and 13C NMR spectroscopy, all the characteristic absorption bands and signals were detected.
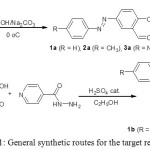 |
Scheme 1: General synthetic routes for the target receptors Click here to View scheme |
Having conveniently synthesized the target compounds 1b–3b, they were subjected to test their affinity and the selectivity toward different anions. Solution of each one were mixed with solutions containing 8 equivalents of the sodium salts of F−, H2PO4−, NO2−, NO3−, Cl−, HSO4− and AcO− at room temperature. The results showed that the sensor 3b with electron withdrawing –NO2 group reacts selectively with AcO− producing a significant color change when the solution turned from clear pale yellow to violet as shown in Figure 1, while all the others did not show any observable color change.
 |
Figure 1: Color changes of 3b solutions (1.35*10−3 M in 9:1 DMSO:H2O) before and after addition of 8 equivalents of sodium salts of various anions. Click here to View figure |
The UV-Vis spectra were recorded before and after addition of the 8 equivalents of the anions to the solution of 3b, it was found that only the addition of AcO− causes a significant increment in the absorption at the visible region with λmax of 535 nm accompanying with decrement of the absorption at 326 nm the λmax of 3b. Very slight increments in the absorption were observed in the visible area of the spectra up on the addition of the other anions, however no significant color change where observed, which confirms the selectivity of the naked-eye detection of 3b toward the AcO−, Figure 2.
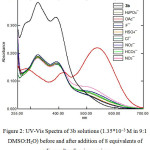 |
Figure 2: UV-Vis Spectra of 3b solutions (1.35*10−3 M in 9:1 DMSO:H2O) before and after addition of 8 equivalents of sodium salts of various anions. Click here to View figure |
The observed color change could be attributed to the deprotonation of the hydroxyl group of the sensor 3b up on the addition of acetate or to the hydrogen bonding between 3b and the AcO− in the manner shown in Scheme 2, resulting a change in electronic properties of the chromophore and producing the violet color. The π delocalization is\ enhanced by the hydrogen bonding leading to the reduction of energy of the π–π* transition which causes the observed bathochromic shift in company with CT (charge transfer) interaction between acetate, –NH, –OH and electron poor –NO2 group of 3b.(29) This explains why only the sensor 3b which has electron withdrawing group by resonance (–NO2) gives positive result in the naked-eye detection of AcO−, the –NO2 contributes in the enhancement of π delocalization and stabilize the resulting complex.
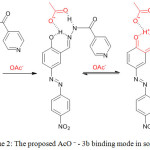 |
Scheme 2: The proposed AcO – – 3b binding mode in solution. Click here to View scheme |
To evaluate the dissociation constant for AcO − with 3b, spectrophotometric titration experiments were performed by recording the UV-Vis spectra for solutions containing 3b and AcO– (0–8 equivalents) using (9:1, DMSO:H2O) as solvent at room temperature, 8 equivalents were required to reach the end point and dissociation constant log βA−of 4.279 was determined for 3b, Figure 3.
![Figure 3: Spectrophotometric titration data: (A) UV-Vis spectra of 3b solutions up on addition of 0 – 8 equivalents of AcO − in 9:1 DMSO:H2O, (B) −log [A−] vs. log [(Amax − A)/ (A − Amin)] at 535nm](http://www.orientjchem.org/wp-content/uploads/2017/11/Vol33No6_Sel_Saj_fig3-150x150.jpg) |
Figure 3: Spectrophotometric titration data: (A) UV-Vis spectra of 3b solutions up on addition of 0 – 8 equivalents of AcO − in 9:1 DMSO:H2O, (B) −log [A−] vs. log [(Amax − A)/ (A − Amin)] at 535nm Click here to View figure |
Conclusions
In conclusion, we reported a new molecular sensor for the selective naked-eye detection of the acetate anion. The sensor 3b, derived from isonicotinohydrazide with imine (Schiff base) binding site and azo signaling unit, interacted with the acetate to produce violet colored solution due to the formation of stable complex with a relatively high dissociation constant log βA−.
Acknowledgement
We thank Al-Qadisiyah University and University of Babylon for the financial support.
References
- Orojloo, M.; Amani, S. C R Chim. 2017, 20, 415-423.
CrossRef - Satheshkumar, A.; El-Mossalamy, E.; Manivannan, R.; Parthiban, C.; Al-Harbi, L.; Kosa, S.; Elango, K. P. Spectrochim. Acta, Part A 2014, 128, 798-805.
CrossRef - Tsui, Y.-K.; Devaraj, S.; Yen, Y.-P. Sens. Actuators, B 2012, 161, 510-519.
CrossRef - Park, S.; Hong, K.-H.; Hong, J.-I.; Kim, H.-J. Sens. Actuators, B 2012, 174, 140-144.
CrossRef - Kaur, N.; Dhaka, G.; Singh, J. Tetrahedron Lett. 2015, 56, 1162-1165.
CrossRef - Dalapati, S.; Alam, M. A.; Jana, S.; Karmakar, S.; Guchhait, N. Spectrochim. Acta, Part A 2013, 102, 314-318.
CrossRef - Dalapati, S.; Jana, S.; Guchhait, N. Spectrochim. Acta, Part A 2014, 129, 499-508.
CrossRef - Farrugia, K. N.; Makuc, D.; Podborska, A.; Szaciłowski, K.; Plavec, J.; Magri, D. C. Eur. J. Org. Chem. 2016, 2016, 4415-4422.
CrossRef - Nie, H.-m.; Gong, C.-b.; Tang, Q.; Ma, X.-b.; Chow, C.-f. Dyes Pigm. 2014, 106, 74-80.
CrossRef - Mohammadi, A.; Yaghoubi, S. Sens. Actuators, B 2017, 241, 1069-1075.
CrossRef - Wang, Z.; Wu, Q.; Li, J.; Qiu, S.; Cao, D.; Xu, Y.; Liu, Z.; Yu, X.; Sun, Y. Spectrochim. Acta, Part A 2017, 183, 1-6.
CrossRef - Nemati, M.; Hosseinzadeh, R.; Zadmard, R.; Mohadjerani, M. Sens. Actuators, B 2017, 241, 690-697.
CrossRef - Sakai, R.; Satoh, T.; Kakuchi, T. Polym Rev 2017, 57, 159-174.
CrossRef - Bao, X.; Zhou, Y. Sens. Actuators, B 2010, 147, 434-441.
CrossRef - Hu, Y.; Li, Y.; Joung, J. F.; Yin, J.; Park, S.; Yoon, J.; Hyun, M. H. Sens. Actuators, B 2017, 241, 224-229.
CrossRef - Saikia, E.; Borpuzari, M. P.; Chetia, B.; Kar, R. Spectrochim. Acta, Part A 2016, 152, 101-108.
CrossRef - Ryu, H. H.; Lee, Y. J.; Kim, S. E.; Jo, T. G.; Kim, C. J Incl Phenom Macrocycl Chem 2016, 86, 111-119.
CrossRef - Ali, H. D. P.; Kruger, P. E.; Gunnlaugsson, T. New J. Chem. 2008, 32, 1153-1161.
CrossRef - Hijji, Y. M.; Barare, B.; Kennedy, A. P.; Butcher, R. Sens. Actuators, B 2009, 136, 297-302.
CrossRef - Guizouarn, H.; Gabillat, N.; Motais, R.; Borgese, F. J. Physiol. 2001, 535, 497-506.
CrossRef - Su, H.; Li, J.; Lin, H.; Lin, H. J. Braz. Chem. Soc. 2010, 21, 541-545.
CrossRef - Gupta, V. K.; Goyal, R. N.; Sharma, R. A. Talanta 2008, 76, 859-864.
CrossRef - Joo, T. Y.; Singh, N.; Lee, G. W.; Jang, D. O. Tetrahedron Lett. 2007, 48, 8846-8850.
CrossRef - Yu, X.; Lin, H.; Cai, Z.; Lin, H. Tetrahedron Lett. 2007, 48, 8615-8618.
CrossRef - Dinçalp, H.; Toker, F.; Durucasu, I.; Avcıbaşı, N.; Icli, S. Dyes Pigm. 2007, 75, 11-24.
CrossRef - Kaur, K.; Singh, A.; Singh, N. Sens. Actuators, B 2014, 191, 734-740.
CrossRef - Huang, W.; Li, Y.; Lin, H.; Lin, H. Spectrochim. Acta, Part A 2012, 86, 437-442.
CrossRef - Farrugia, K. N.; Makuc, D.; Podborska, A.; Szaciłowski, K.; Plavec, J.; Magri, D. C. Org Biomol Chem. 2015, 13, 1662-1672.
CrossRef - Goswami, S.; Das, A. K.; Sen, D.; Aich, K.; Fun, H.-K.; Quah, C. K. Tetrahedron Lett. 2012, 53, 4819-4823.
CrossRef

This work is licensed under a Creative Commons Attribution 4.0 International License.









