Production Methods of Iodine-doped Carbon Nano Tubes (A Review)
E. Yu. Melnikova, V. N. Glushko, V. S. Ivanov and M. Yu. Zhila
The Federal State Unitary Enterprise «Institute of Chemical Reagents and High Purity Chemical Substances of National Research Centre «Kurchatov Institute» 107076, Bogorodsky val, 3, Moscow, Russia.
Corresponding Author E-mail: margo.yu@gmail.com
DOI : http://dx.doi.org/10.13005/ojc/330603
This review considers various methods of doping single- and multi-walled carbon nanotubes (CNT) with iodine and iodine derivatives to improve nanotube properties, and describes advantages and disadvantages of each of presented methods. It also describes methods of purifying obtained samples.
KEYWORDS:Carbon Nanotubes; Iodine-Doping; Iodine Monochloride; Sublimational Sedimentation; Hunsdiecker Reaction
Download this article as:| Copy the following to cite this article: Melnikova E. Y, Glushko V. N, Ivanov V. S, Zhila M. Y. Production Methods of Iodine-doped Carbon Nano Tubes (A Review). Orient J Chem 2017;33(6). |
| Copy the following to cite this URL: Melnikova E. Y, Glushko V. N, Ivanov V. S, Zhila M. Y. Production Methods of Iodine-doped Carbon Nano Tubes (A Review). Orient J Chem 2017;33(6). Available from: http://www.orientjchem.org/?p=40244 |
Introduction
Nowadays chemical modification of single- and multi-walled carbon nanotubes is one of rapidly expanding and important field of scientific research. Various methods of functionalization, intercalation, doping and other numerous treatment methods are designed to improve optical, electrical, chemical and mechanical properties of both nanotubes and wires, films and layers made of nanotubes.
At the present time halogens are increasingly considered as CNT intercalators. They are paid particular attention due to the fact that they have high reactivity and easily integrate into CNT structure (Figure 1). According to literature data1, iodination or bromination are known to specifically affect CNT, since such doping produces р-type semiconductors. However, there is an issue of stability of chlorine- (Cl2) or bromine- (Br2) modified CNT: such CNT are unstable in the air at room temperature. On the contrary, iodine-modified CNT show resistance under any conditions without losing their conducting properties. Another reason for choosing iodine for CNT doping is significantly low sublimation point (transition from solid state to gaseous one takes place at 114.5˚С), which allows to avoid the destruction of crystal structure of CNT used for doping.
Methods of CNT Iodine-Doping
There are currently several methods of doping CNT with various substances: nanotubes doping during their growth (i.e. in situ methods); gas or liquid phase incapsulation in the cavity of preliminary formed CNT and chemical modification of CNT surface (i.e. ex situ methods)3.
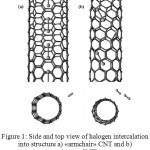 |
Figure 1: Side and top view of halogen intercalation into structure а) «armchair» CNT and b) «zigzag» CNT2 |
In situ methods include CNT modification during arc-discharge synthesis and CVD method. The main disadvantage of the in situ strategy to fill single-layer nanotubes is its low efficiency: the output of filled SWCNT does not exceed few percentage points3.
Ex situ melt incapsulation has a number of advantages over other doping methods. They include a possibility of using a wide range of substances to fill CNT, simple approach, uniformity of composite materials, high load rate (up to 90%) and high crystallinity of synthesized nanoparticles. Another advantage is the absence of contamination of solvents and/or by-products (oxides and carbides) in the «1D-crystall/SWCNT» systems. Gas phase intercalation allows to control the process of filling CNT with a substance. Hence ex situ methods are considered to be the most effective ones, comparing to in situ ones3. of those mentioned above, scientific literature describes three methods of doping carbon nanotubes with iodine. The first one is sublimational sedimentation, the second one is CNT treatment in melted iodine, and the third one is Hunsdiecker reaction using oxidized CNT.
Sublimational Sedimentation Method
Numerous studies of Russian research group led by E.D. Obrazsovsa describe the method of iodine-modifying single-walled carbon nanotubes (SWCNT) by chemical gas-phase filling SWCNT channels1,4,5. This method allows to avoid the destruction of crystal structure of SWCNT being used. However, this method is featured by low filling rate.
SWCNT samples applied to quartz or silicon plates as thin films were used for modification. For this purpose the following types of SWCNT were used: 1) grown by catalytic method, using Co and Mo, with their diameter range of 0.6 to 1.3 nm («СоМоcat method»); 2) produced by high-pressure СО decomposition with their diameter range of 0.8 to 1.5 nm («HipCO method»); and 3) grown by aerosol chemical CVD method with their diameter range of 1.3 to 2.0 nm.
The process of filling SWCNT with iodine atoms comprises several stages. First, SWCNT were prepared, i.e. the films were formed and applied to the surfaces of quartz or silicon plates. Organic impurities were removed from film samples by in-the-air preheating (2 h, 150-200°С). Second, the doping was carried out in the chemical reactor, a durable glass bin put inside tube induction furnace. The temperature of filling SWCNT with iodine (120-130°С) was chosen to be as similar as possible to that of iodine sublimation (114.5°С). Such range (120-130°С) contributes to effective capillary condensation and iodine liquification inside SWCNT’s own pores (open tube ends and natural growth defects). Higher temperatures reduce the efficiency of filling nanotube pores. During the modification SWCNT, used as nanocontainers of iodine structures, were not exposed to additional impact aimed at creating a large number of pore defects, to improve the efficiency of iodine penetration into SWCNT channels. In case of «СоМоcat» and «HipCO» SWCNT, sufficient number of pores is ensured by the processes of chemical removal of amorphous carbon and catalyst particles, which are natural products of nanotube growth in these methods, from these nanotubes. SWCNT produced by aerosol chemical method were not subjected to purification processes, since this method allows to produce especially pure material that virtually contain neither amorphous carbon nor free catalyst particles. Thus, iodine reduction occurs at open tube ends, formed after chemical removal during purification or at points of SWCNT’s natural breakage, and also at points of natural defects and defects that occur during purifying SWCNT walls. The filling was carried out in a static air environment for 12 hours, without additional pressure adjustment. The final stage was reactor cleaning and sample purification. This stage took place at the same temperature in the dynamic vacuum (about 10-2 mbar, 4 hours) and led to complete removal of gaseous and physically adsorbed iodine1. Sometimes the authors carried out additional purification of doped SWCNT by ultrasonic treatment. This process allows removing polyiodides from the cavities between nanotube bundles4.
The essence of this method can be described as follows. For the purpose of iodine intercalation, the films are applied to quartz or silicon trays. Then, organic impurities are removed from the samples by in-the-air preheating in the electric furnace (2 h, 150-200°С). Iodination is carried out in the gas phase. Reactor’s glass chamber, which contains crystal iodine (Sigma-Aldrich, purity: over 99.8%) and SWCNT, is heated to 125-130°C. The duration of intercalation is about 12 hours. After that the samples and reactor are subjected to thermal 4-hours purification /cleaning at the temperature mentioned above5.
There is another interpretation of sublimational sedimentation method developed by American scientists6.
Nanotubes, produces by «HipCO» method, were used as initial material for the study. Average diameter of sample’s nanotubes is 1.0 nm, while the content of iron impurities is about 35 %wt. The sample was not additionally purified with strong acid, which is known to both remove iron impurities and create additional defects in side walls.
SWCNT sample was filled using I2 sublimation (about 100°С) while being kept in enclosed glass vessel for an hour. After completion of the doping process excessive iodine was removed from SWCNT surface in two ways. The first way was conducting reduction reaction using Na0/THF, while the second way was 300°С thermal treatment without additional chemical treatment. Both ways produced iodine-doped SWCNT with iodine content of about 25 %wt. The authors note that 300°С thermal treatment is a preferable way of purification, since SWCNT material is not contaminated by foreign substances.
Method of CNT Doping in Melted Iodine
Method of doping single-walled carbon nanotubes in melted iodine was described by American scientists as early as in 19987.
The authors of article7 developed a simple method of SWCNT iodine-doping. Intercalation was carried out by dipping SWCNT layers (benches) into melted iodine inside vacuumized quartz tubes at 140°С for several hours. SWCNT, produced by laser and arc methods, were used as initial doping material. In terms of the amount of iodine added, commensurable results were obtained for both SWCNT samples. For the purpose of annealing doped fiber structure and removing excessive physically adsorbed iodine, doped layers were subjected to in situ thermal treatment in the following way: quartz tube end containing doped SWCNT was heated to 60-80°С for 2-4 for hours, while the other end was dipped into liquid nitrogen to collect excessive iodine.
In 1999 the same research group complemented previously developed melted-iodine modification method and took out a patent for it8. This method means dipping single-walled nanotubes into melted iodine and soaking it at 80-200°С (preferable temperature of reaction environment: 140-160˚С) for 0.5 – 10 hours.
This process was conducted inside sealed vacuumized (up to 10-2 torr) reactor tube made of Pyrax glass. Upper temperature limit was chosen on the basis of iodine melting point and melted iodine viscosity at a given temperature. Upper temperature limit was also limited by glass reactor walls’ resistance to higher pressure of iodine fumes. Lower temperature limit (80°С) was due to low efficiency of SWCNT iodine-doping. At Т<80°С SWCNT filling takes several days instead of several days at 140-160°С.
To remove all excessive unreacted iodine, the reactor end, containing doped sample, was then heated to 80-100°С for 12-24 hours, while the other end was cooled with liquified nitrogen to collect gaseous iodine. The process was deemed complete, when iodine desublimation was no longer observable (it is easy to find out by the absence of intense coloring of pink iodine steam), followed by extracting sample from the reactor.
In early 2000s the above-mentioned method of SWCNT doping in melted iodine was applied to multi and double-walled carbon nanotubes. As shown in articles9,10, this method is suitable for iodine-doping of any carbon nanotubes, thus being universal.
Multi-walled carbon nanotubes (MWCNT), used in study9, were obtained by arc discharge method. Then they were oxidized in the air at 700°С for an hour to open nanotube ends. Scanning electron microscopy revealed that on average produced MWCNT consist of 15 layers, while their inner diameter varied from 0.5 to 7.5 nm, with the average value of 3.66 nm. Intercalation was carried out by dipping MWCNT into melted iodine inside vacuumized quartz tube at 140˚С for about one week. To remove excessive physically adsorbed iodine, the samples were thermally treated in the following way. Quartz tube end, containing doped MWCNT, was heated to 70°С for 5-6 hours, the other end was dipped into liquid nitrogen to collect excessive iodine.
The authors of article11 used double-walled carbon nanotubes (DWCNT) produced by chemical vapor deposition (CVD method). Then amorphous carbon and catalysis impurities were removed from the nanotubes by multi-stage oxidation. DWCNT were first oxidized by heating raw macroscopic DWCNT bundle in the air at 400°C for an hour. Than oxidized DWCNT were soaked in 30% hydrogen peroxide solution for 72 hours. This soaking process allows to crack amorphous carbon and makes catalyst dissociate from carbon nanotubes. After that DWCNT were transferred into 37% hydrogen chloride solution to soak it for another 24 hours. DWCNT, produced by the above-mentioned procedure, was washed with distilled water till washing liquid turned neutral. The content of catalyst after such purification was less than 1%. This purification resulted in DWCNT bundles. To separate these bundles, they were loosened and wrapped into thin films to be soaked in 98% sulfuric acid for 24 hours. Once the soaking was completed, a small amount of belt-shaped DWCNT was extracted from thin films. Then the belts were flushed with water to remove residual sulfuric acid. Finally, produced belts were put together into bundles of a given diameter.
It was those treated DWCNT bundles that were used for iodine-doping. This process was conducted by exposing DWCNT bundles to iodine fumes (concentration of iodine fumes: 0.2 mol/l) at 200°С for 12 hours.
CNT Doping by Chemical Treatment Method
In 2006 British researchers developed a method of modifying oxidized CNT using Hunsdiecker reaction12. The authors of the article used single-walled carbon nanotubes (SWCNT) produced by «HipCO» method.
Prior to commencing its use, SWCNT was prepared as follows: metal particle impurities were removed and purified SWCNT were annealed in vacuum environment (10-2 mbar) at 1000°С for 2 hours. The authors adopted SWCNT purification process mentioned in article13. Unpurified tubes, produced by «HipCO» method, were oxidized in humid Ar/O2 environment at various temperatures. Low-density (about 0.01 g/cm3) raw nanotubes, obtained by «HipCO» method, were pinned to dry surface of filter paper, placed over glass filter, by vacuum. This approach prevents nanotube material from scattering. Then SWCNT (about 100 mg) were put into ceramic cup and placed into the furnace containing quartz tube. Gas mixture, containing 20% of О2 in Ar (using air is acceptable), was forced to run through the water by air bubbler and fed to the furnace with its volume flow rate of 100 cm3/min. Nanotubes were exposed at 225°С for 18 hours and then either treated with ultrasound for 15 min or mixed in the concentrated HCl solution for a long period of time (during night). After that, the tubes, exposed to acid solution, were filtered on 47-mm Teflon diaphragm with its pore size of 1 µm (Cole-Parmer) and washed with deionized water and methanol several times. Then they were dried in the vacuum furnace at 100°С for an hour. Oxidation and acid extraction cycle was repeated at 325˚С and 425°С for 1.5 hours and an hour respectively. Upon completion of drying in the vacuum furnace, «HipCO» tubes were annealed in Ar at 800°С for an hour.
After thorough purification SWCNT were oxidized by suspending their material with 6 М HNO3 and boiling for 17 hours. The product was separated by centrifugation (4000 rpm for 30 minutes), followed by removing supernatant fluid solution and adding deionized water into centrifugal tubes. Centrifugation was repeated till supernatant fluid solution turned neutral. Then oxidized SWCNT material (SWCNT-СООН) was extracted by filtering (0.2-µm polycarbonate diaphragm) and dried in the furnace at 80°С overnight.
After that, oxidized SWCNT, SWCNT-СООН (10 mg), were suspended in the 80-W ultrasonic bath without temperature control using СCl4 (100 ml). Then 0.161 g (0.5 mmol) of iodosobenzene diacetate (IBDA) and 0.127 g (0.5 mmol) of iodine were added. Reaction substance was boiled and mixed simultaneously for an hour, while being illuminated by 200-W tungsten filament lamp. In an hour another 0.161 g (0.5 mmol) of IBDA and 0.127 g (0.5 mmol) of iodine were added to the reaction mixture, followed by 15-hour boiling under illumination. After that the reaction mixture was cooled to the room temperature, followed by adding 0.79-g (5 mmol) Na2S2O3 solution in deionized water (50 ml). Once the purple reaction solution turned colorless, solid product was filtered (polycarbonate diaphragm, pore diameter: 0.2-µm). The product was washed using 2х25-ml 0.2-µm with Na2S2O3 solution in deionized water, followed by 4х50-ml deionized water and 1х20-ml ethanol. The product was dried at 80°С overnight (Figure 2).
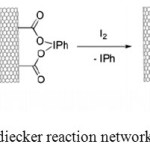 |
Figure 2: Modified Hunsdiecker reaction network exemplified by SWCNT |
Use of Iodine Derivatives for CNT Doping
Scientific literature contains methods of modifying carbon nanotubes with iodine derivative. Single-, double- and multi-walled CNT were doped with polar interhalogen compound, iodine monochloride (ICl)14.
American researchers produced cables based on different-walled (single-, double- and multi-walled) CNT using CVD furnace. Then these cables were doped in two ways. The first way was to expose CNT bundles to ICl at room temperature for 8 hours. The second way was short-term self-heating of CNT bundle over iodine monochloride to 350˚С, using Joule–Thomson effect (self-heating under slow gas flow through porous baffle caused by constant pressure differential). The second way proved unsuitable for doping, since it led to partial destruction of the material. This modification resulted in producing bundles of p-type conductance. As the studies showed, ICl doping allows to reduce material’s electrical resistance by over 60%, which results in specific electrical conductivity of 1.24 Sm/m.
Acknowledgments
The work was supported by the Russian Federation Ministry of Education and Science under the grant agreement № 14.625.21.0037 of 3 October 2016 (the project № RFMEF162516X0037).
References
- Tonkikh, A.A. Optical and electronic properties of iodine-doped single-wall carbon nanotubes. Dissertation, 2013.
- Jhi, S.-H.; Louie, S.G.; Cohen, M.L. Electronic properties of bromine-doped carbon nanotubes. Solid State Comm. 2002, 123, 495-499.
CrossRef - Eliseev, A.; Yashina, L.; Kharlamova, M.; Kiselev, N. One-dimensional crystals inside single-walled carbon nanotubes: growth, structure and electronic properties. J.M. Marulanda (Ed.), Electronic Properties of Carbon Nanotubes, In-Tech Croatia, 2011, 127-156.
- Tonkikh, A.A.; Obraztsova, E.D.; Obraztsova, E.A.; Belkin, A.V.; Pozharov, A.S. Optical spectroscopy of iodine-doped single-wall carbon nanotubes of different diameter. Phys. Status Solidi B, 2012, 249(12), 2454-2459.
CrossRef - Tonkikh, A.A.; Tsebro, V.I.; Obraztsova, E.A.; Suenaga, K.; Kataura, H.; Nasibulin, A.G.; Kauppinen, E.I.; Obraztsova, E.D. Metallization of single-wall carbon nanotube thin films induced by gas phase iodination. Carbon, 2015, 94, 768-774.
CrossRef - Kissell, K.R.; Hartman, K.B.; Van der Heide, P.A.W.; Wilson, L.J. Preparation of I2@SWNTs: Synthesis and Spectroscopic Characterization of I2-Loaded SWNTs. J. Phys. Chem. B, 2006, 110, 17425-17429.
CrossRef - Grigorian, L.; Williams, K.A.; Fang, S.; Sumanasekera, G.U.; Loper, A.L.; Dickey, E.C.; Pennycook, S.J.; Eklund, P.C. Reversible Intercalation of Charged Iodine Chains into Carbon Nanotube Ropes. Phys. Rev. Lett. 1998, 80(25), 5560-5563.
CrossRef - Eklund, P.C.; Grigorian, L.; Williams, K.A.; Sumanasekera, G.U.; Fang, Sh. Metallic Nanoscale Fibers From Stable Iodine-Doped Carbon Nanotubes. US Patent No. US006139919A, 1999.
- Zhou, W.; Xie, S.; Sun, L.; Tang, D.; Li, Yu.; Liu, Z.; Ci, L.; Zou, X.; Wang, G. Raman scattering and thermogravimetric analysis of iodine-doped multiwall carbon nanotubes. Applied Physics Lett. 2002, 80(14), 2553-2555.
CrossRef - Cambedouzou, J.; Sauvajol, J.-L; Raman spectroscopy of iodine-doped double-walled carbon nanotube. Physical Review B, 2004, 69, 235422.
CrossRef - Zhao, Y.; Wei, J.; Vajtai, R.; Ajayan, P.M.; Barrera, E.V. Iodine doped carbon nanotube cables exceeding specific electrical conductivity of metals. Scientific Reports, 2011, 1-6.
CrossRef - Coleman, K.S.; Chakraborty, A.K.; Bailey, S.R.; Sloan, J.; Alexander, M. Iodination of Single-Walled Carbon Nanotubes. Chem. Mater. 2007, 19, 1076-1081.
CrossRef - Chiang, I.W.; Brinson, B.E.; Huang, A.Y.; Willis, P.A.; Bronikowski, M.J.; Margrave, J.L.; Smalley, R.E.; Hauge, R.H. Purification and Characterization of Single-Wall Carbon Nanotubes (SWNTs) Obtained from the Gas-Phase Decomposition of CO (HiPco Process). J. Phys. Chem. B, 2001, 105, 8297-8301.
CrossRef - Janas, D.; Herman, A.P.; Boncel, Sl.; Koziol, Krz.K.K. Iodine monochloride as a powerful enhancer of electrical conductivity of carbon nanotube wires. Carbon, 2014, 73, 225-233.
CrossRef

This work is licensed under a Creative Commons Attribution 4.0 International License.









